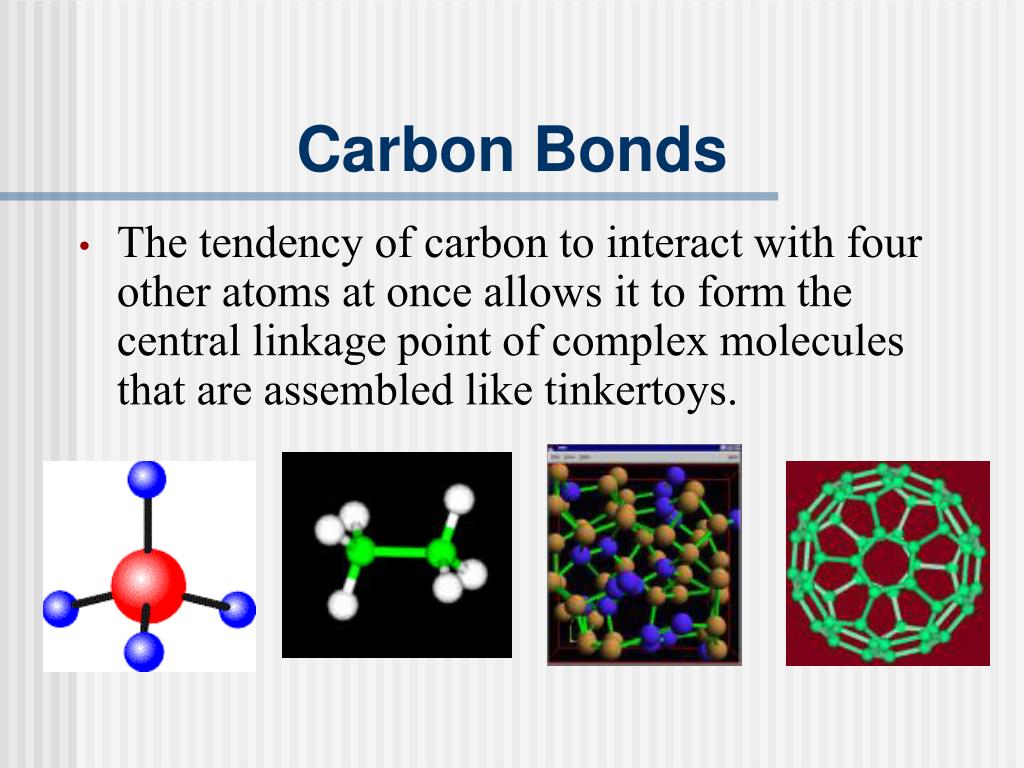
Maximum Number Of Bonds A Carbon Atom Can Form
Maximum Number Of Bonds A Carbon Atom Can Form - And group 7a form one bond. Web moreover, of all the elements in the second row, carbon has the maximum number of outer shell electrons (four) capable of forming covalent bonds. So in order for carbon to have that full set of electrons, it is going to accept four more. Is not restricted by the number of hybrid. Web so, from observation alone, we can conclude that a carbon with two double bonds is possible. Web chemistry chemistry questions and answers see diagram part b what is the maximum number of triple bonds that a carbon atom can form? This problem has been solved! The author explains that carbon binds with hydrogen atoms resulting in. 2)how many hydrogen atoms must bond to silicon to give it an octet of valence electrons? Carbon’s ability to form bonds with four other atoms goes back to its number and configuration of electrons. Web 1)what is the maximum number of double bonds that a carbon atom can form? Web ad organic chemistry provides a wealth of examples where carbon has the four covalent bonds to hydrogen, oxygen, nitrogen, or other atoms.or to carbon. The four covalent bonding positions of the carbon atom can give rise to a wide diversity of compounds with many. What is the maximum number of triple. The author explains that carbon binds with hydrogen atoms resulting in. Web 4 rows the number of electrons required to obtain an octet determines the number of covalent bonds an. This is summarized in the table below. Ad browse & discover thousands of book titles, for less. What is the maximum number of triple. This problem has been solved! So in order for carbon to have that full set of electrons, it is going to accept four more. The situation with calcium is irrelevant as most compounds will not be covalent. You can also look at it this way that a carbon can make four bond. Web chemistry chemistry questions and answers see diagram part b what is the maximum number of triple bonds that a carbon atom can form? Web moreover, of all the elements in the second row, carbon has the maximum number of outer shell electrons (four) capable of forming covalent bonds. 2)how many hydrogen atoms must bond to silicon to give it. Web a full set would be eight. Group 5a form 3 bonds; Web considering the number of other atoms a central atom can be bonded to, hybridization [is] irrelevant and the coordination number. The author explains that carbon binds with hydrogen atoms resulting in. This problem has been solved! 2)how many hydrogen atoms must bond to silicon to give it an octet of valence electrons? The author explains that carbon binds with hydrogen atoms resulting in. You'll get a detailed solution from a subject matter expert that. Is not restricted by the number of hybrid. Web chemistry chemistry questions and answers see diagram part b what is the maximum. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web 1)what is the maximum number of double bonds that a carbon atom can form? So in order for carbon to have that full set of electrons, it is going to accept four more. And group 7a form one bond. You'll get a detailed. Is not restricted by the number of hybrid. This problem has been solved! Carbon’s ability to form bonds with four other atoms goes back to its number and configuration of electrons. Web 4 rows the number of electrons required to obtain an octet determines the number of covalent bonds an. Group 5a form 3 bonds; You'll get a detailed solution from a subject matter expert that. Web so, from observation alone, we can conclude that a carbon with two double bonds is possible. Web a full set would be eight. John’s school vs osei tutu shs vs opoku ware school So in order for carbon to have that full set of electrons, it is going. You'll get a detailed solution from a subject matter expert that. Is not restricted by the number of hybrid. The author explains that carbon binds with hydrogen atoms resulting in. John’s school vs osei tutu shs vs opoku ware school Ad browse & discover thousands of book titles, for less. Web 1)what is the maximum number of double bonds that a carbon atom can form? You'll get a detailed solution from a subject matter expert that helps you learn core concepts. 2)how many hydrogen atoms must bond to silicon to give it an octet of valence electrons? Web the number of electrons required to obtain an octet determines the number of covalent bonds an atom can form. Carbon’s ability to form bonds with four other atoms goes back to its number and configuration of electrons. Web moreover, of all the elements in the second row, carbon has the maximum number of outer shell electrons (four) capable of forming covalent bonds. Web a full set would be eight. The four covalent bonding positions of the carbon atom can give rise to a wide diversity of compounds with many functions, accounting for the importance of. Web 4 rows the number of electrons required to obtain an octet determines the number of covalent bonds an. Web the ways in which an atom can bond with other atoms depends on the atom's ______. Group 5a form 3 bonds; Is not restricted by the number of hybrid. Ad browse & discover thousands of book titles, for less. Web what is the maximum number of double (pi) bonds that a carbon atom can form? Web typically, the atoms of group 4a form 4 covalent bonds; What is the maximum number of triple. You can also look at it this way that a carbon can make four bond. Web chemistry chemistry questions and answers see diagram part b what is the maximum number of triple bonds that a carbon atom can form? John’s school vs osei tutu shs vs opoku ware school Web so, from observation alone, we can conclude that a carbon with two double bonds is possible.How to Predict number of bonds each element forms ChemSimplified
The 4 Types of Bonds Carbon Can Form Video & Lesson Transcript
Carbon to Carbon Single, Double & Triple Bonds Surfguppy
PPT Biochemistry PowerPoint Presentation, free download ID89333
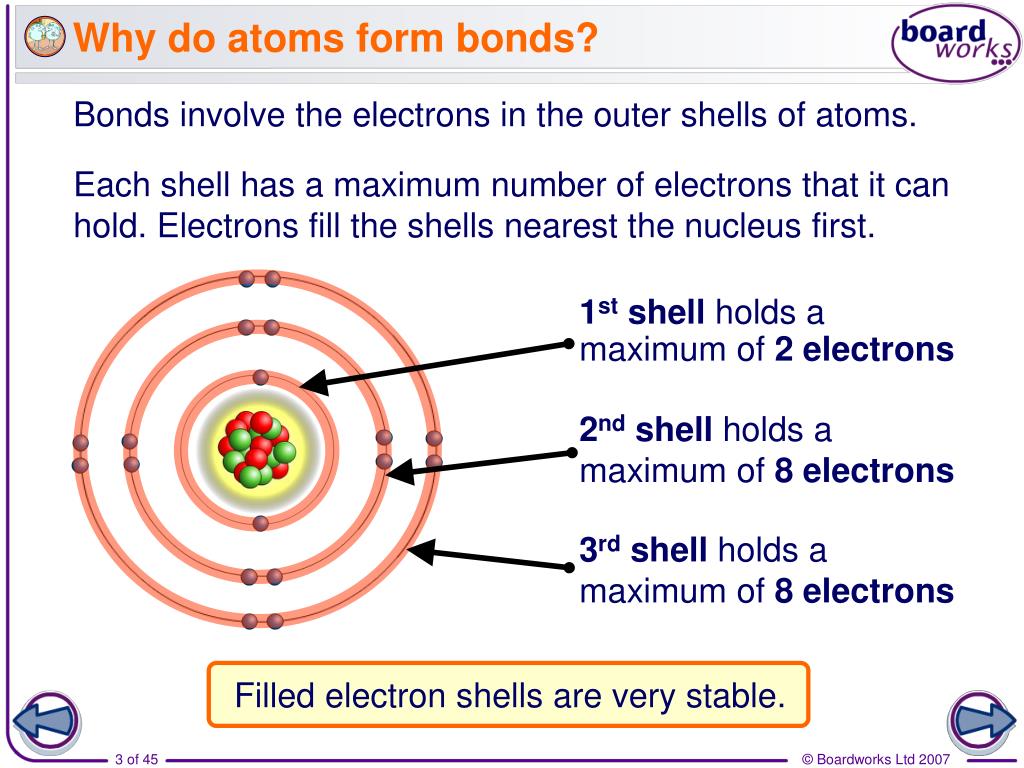
PPT Why do atoms form bonds? PowerPoint Presentation, free download
PPT Biochemistry PowerPoint Presentation, free download ID89333
PPT Unit 1 Biochemistry The Chemistry of Life PowerPoint
What is the maximum number of triple bonds that a carbon atom can form
Solved What Is The Maximum Number Of Bonds That The Atom
PPT Carbon Compounds PowerPoint Presentation, free download ID2319022
Related Post: