Which Pair Of Compounds Will Form A Solution
Which Pair Of Compounds Will Form A Solution - You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Nacl and hexane (csh14) d. Which pairs of compounds will form a. Web the pair of compounds that will form a solution is h2o and nacl. Choose the pair of substances that are most likely to form a. Web which pairs of compounds would you expect to form homogeneous solutions when combined? Web which pair of compounds will form a solution? Asked 5 years, 5 months ago. Terms in this set (117) which of the following pairs of compounds would you expect to form homogeneous solutions when. Web precipitates are insoluble ionic solid products of a reaction, formed when certain cations and anions combine in an aqueous solution. Web which pair of compounds will form a solution? For those that form homogeneous solutions, indicate the type of forces that. Web which pair of compounds will form a solution? Web which pair of compounds will form a solution? Nacl and hexane (c6h14) na2so4 and. Nacl and hexane (csh14) d. A) ch3ch2ch2ch2ch3 and ch3ch2ch3 b) nacl and h2o c). Web study with quizlet and memorize flashcards containing terms like according to the solubility principle of like dissolves like, which pair of compounds will generally form a solution?,. For those that form homogeneous solutions, indicate the. Web which of the following pairs of compounds would you. Web how to determine which ions will pair to form a compound in an aqueous solution? In order to determine which pair of compounds will form a solution, we. A) ch3ch2ch2ch2ch3 and ch3ch2ch3 b) nacl and h2o c). A benzene (c6h6) and hexane (c6h14) b na2so4 and benzene (c6h6) c nacl and hexane (c6h14) d h2o and ccl4. More than. In order to determine which pair of compounds will form a solution, we. What kind of forces would it/they form? A) ch3ch2ch2ch2ch3 and ch3ch2ch3 b) nacl and h2o c). Web which pair of compounds will form a solution? Web precipitates are insoluble ionic solid products of a reaction, formed when certain cations and anions combine in an aqueous solution. Web which pair of compounds will form a solution? Web when particles with no charges (nonpolar compounds) are mixed, they will form a solution. However, if substances with charges are mixed with other substances. C) n a c l and hexane. Web how to determine which ions will pair to form a compound in an aqueous solution? Modified 5 years, 5 months ago. Web which pair of compounds will form a solution? Benzene (cgh6) and hexane (c6h14) b. Group of answer choices more than one of the combinations above will form solu… Nacl and hexane (csh14) d. Web which of the following pairs of compounds would you expect to form homogeneous solutions when combined? Benzene (cgh6) and hexane (c6h14) b. C) n a c l and hexane. Modified 5 years, 5 months ago. Determine whether each pair of compounds forms a homogeneous solution when combined. B) n a 2 s o 4 and benzene. Web which of the following pairs of compounds would you expect to form homogeneous solutions when combined? Web compounds like sodium stearate, called 'surfactants' in general, can form structures known as micelles in wate. More than one of the combinations above will form solutions. A) benzene (c6h6) and hexane (c6h14) b). Choose the pair of substances that are most likely to form a. Web find an answer to your question which pair of compounds will form a solution? For those that form homogeneous solutions, indicate the. Hi, please helpwhich pairs of compounds form a homogenous solution? A benzene (c6h6) and hexane (c6h14) b na2so4 and benzene (c6h6) c nacl and hexane. More than one of the combinations above will form solutions. Nacl and hexane (csh14) d. Web the pair of compounds that will form a solution is h2o and nacl. Group of answer choices more than one of the combinations above will form solu… Web when particles with no charges (nonpolar compounds) are mixed, they will form a solution. Web which of the following pairs of compounds would you expect to form homogeneous solutions when combined? You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web how to determine which ions will pair to form a compound in an aqueous solution? Web when particles with no charges (nonpolar compounds) are mixed, they will form a solution. Web the pair of compounds that will form a solution is h2o and nacl. Web find an answer to your question which pair of compounds will form a solution? Web study with quizlet and memorize flashcards containing terms like according to the solubility principle of like dissolves like, which pair of compounds will generally form a solution?,. Web which pair of compounds will form a solution? A) benzene (c6h6) and hexane (c6h14) b) na2so4 and benzene (c6h6) c) nacl and hexane (c6h14) d) h2o and ccl4 e) more. C) n a c l and hexane. Web compounds like sodium stearate, called 'surfactants' in general, can form structures known as micelles in wate. Which pairs of compounds will form a. Nacl and hexane (csh14) d. Web which pair of compounds will form a solution? More than one of the combinations above will form solutions. For those that form homogeneous solutions, indicate the type of forces that. Benzene (cgh6) and hexane (c6h14) b. A) ch3ch2ch2ch2ch3 and ch3ch2ch3 b) nacl and h2o c). Web which pair of compounds will form a solution? Asked 5 years, 5 months ago.Solved Which pair of compounds will form a buffer in aqueous
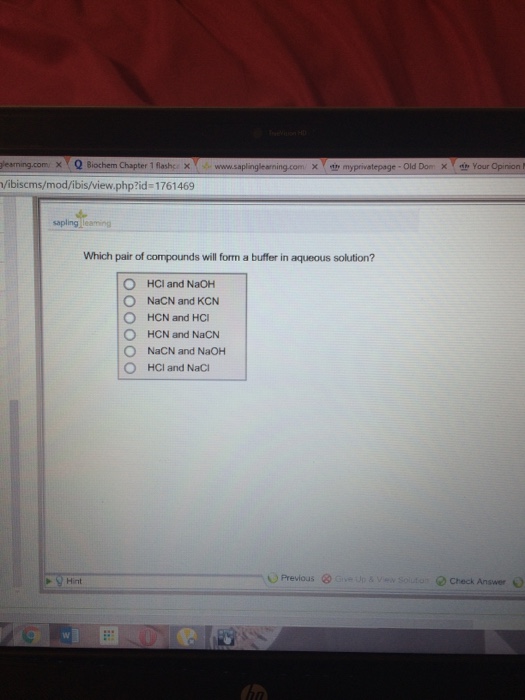
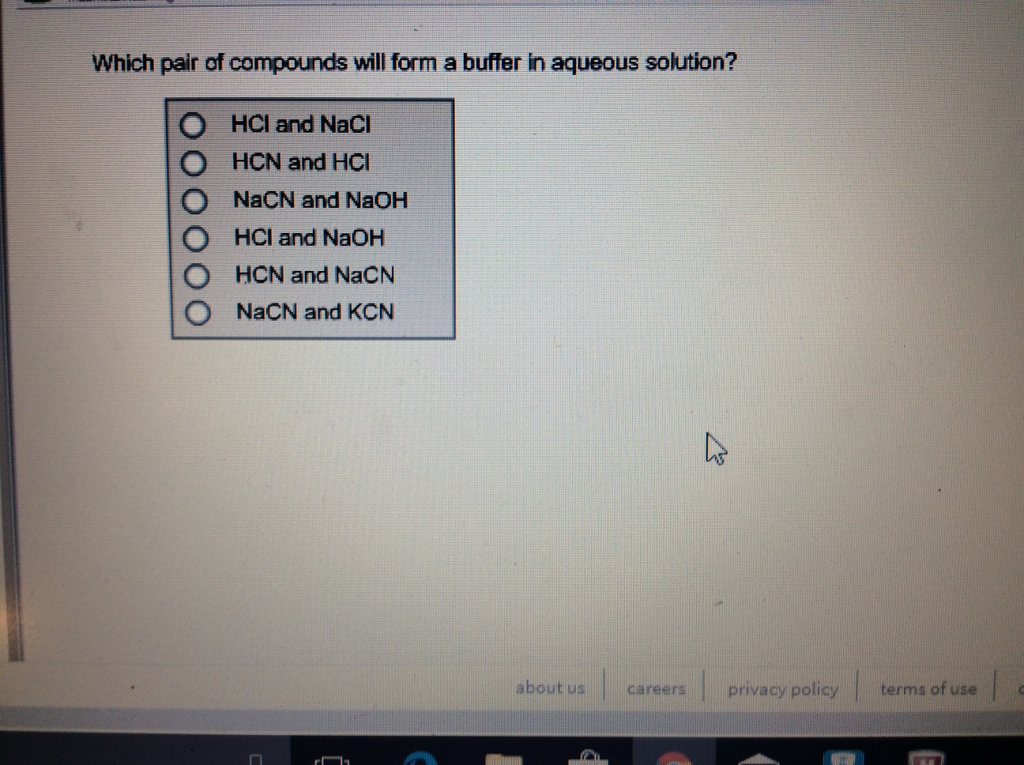
Which pair of compounds will form a buffer in an aqueous solution? a
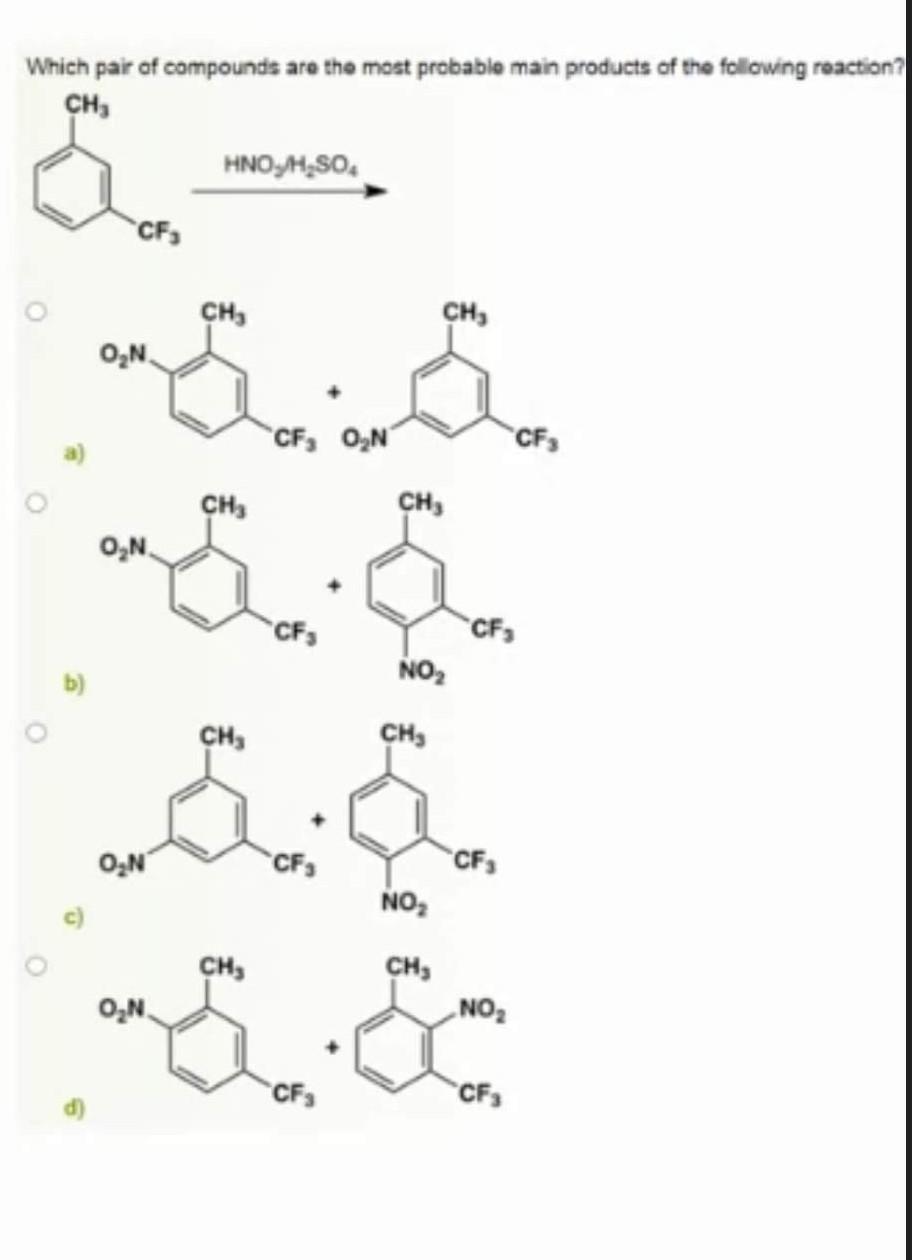
Solved Which pair of compounds are the most probable main
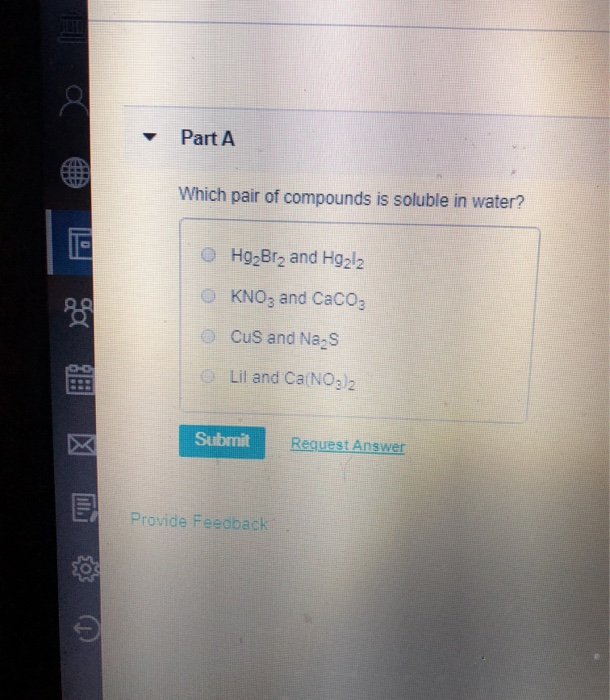
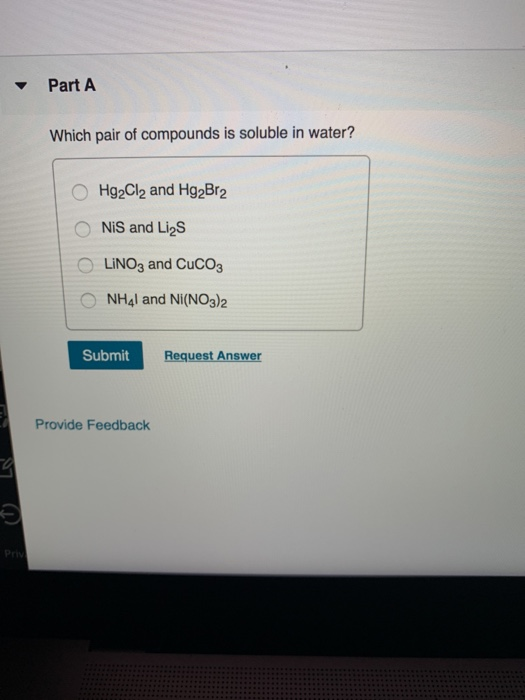
Solved Part A Which pair of compounds is soluble in water?
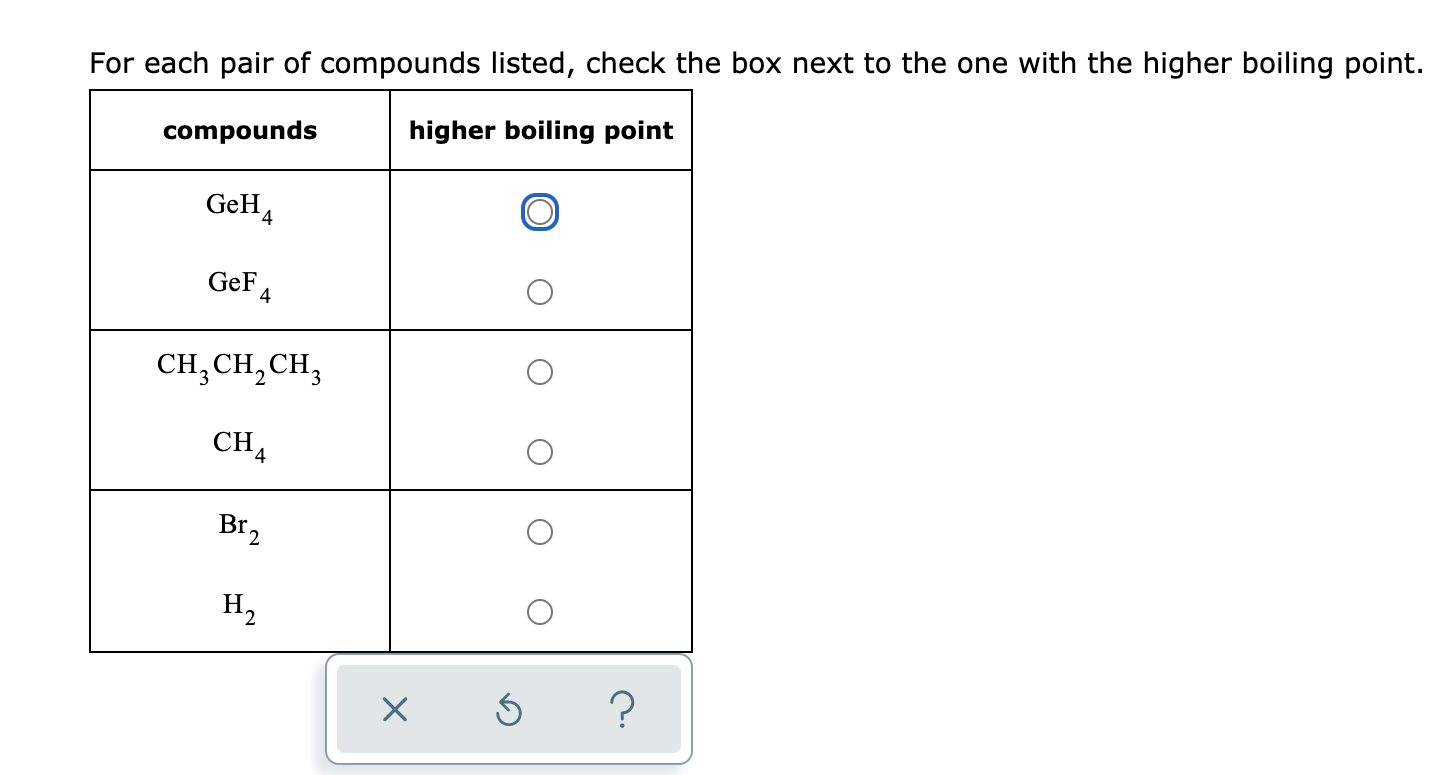
Solved For each pair of compounds listed, check the box next
Solved Part A Which pair of compounds is soluble in water?
Solved Which pair of compounds will form a buffer in aqueous
SOLVEDWhich of the following pairs of compounds have the same
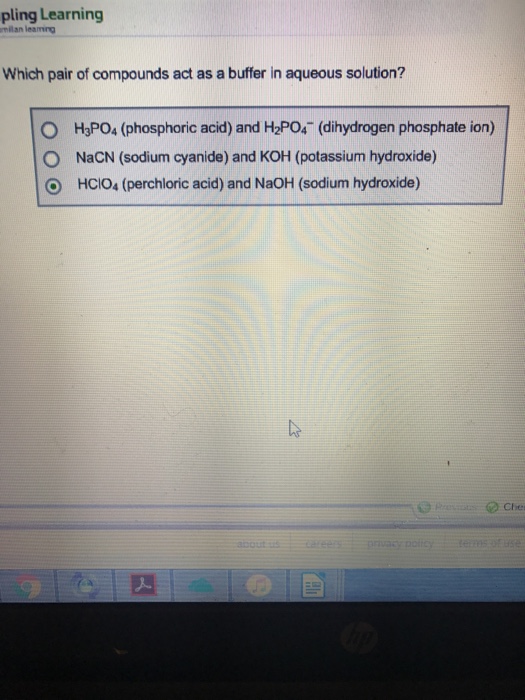
Solved Which Pair Of Compounds Act As A Buffer In Aqueous...
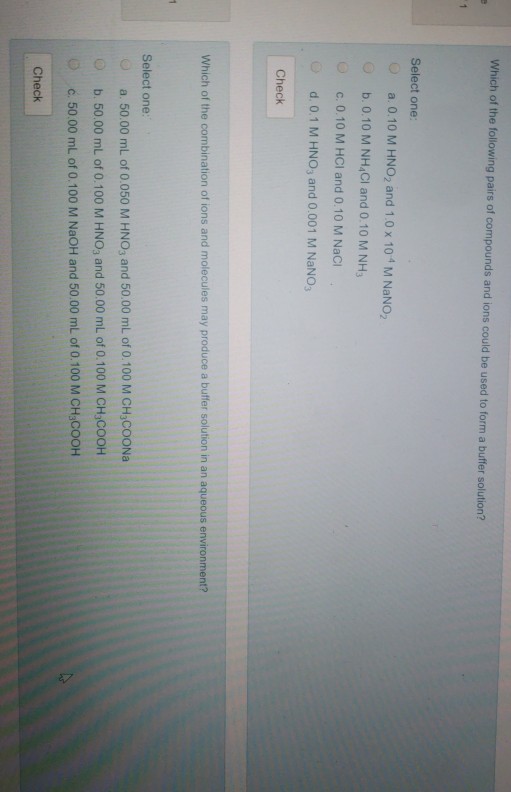
Solved Which of the following pairs of compounds and ions
Related Post: