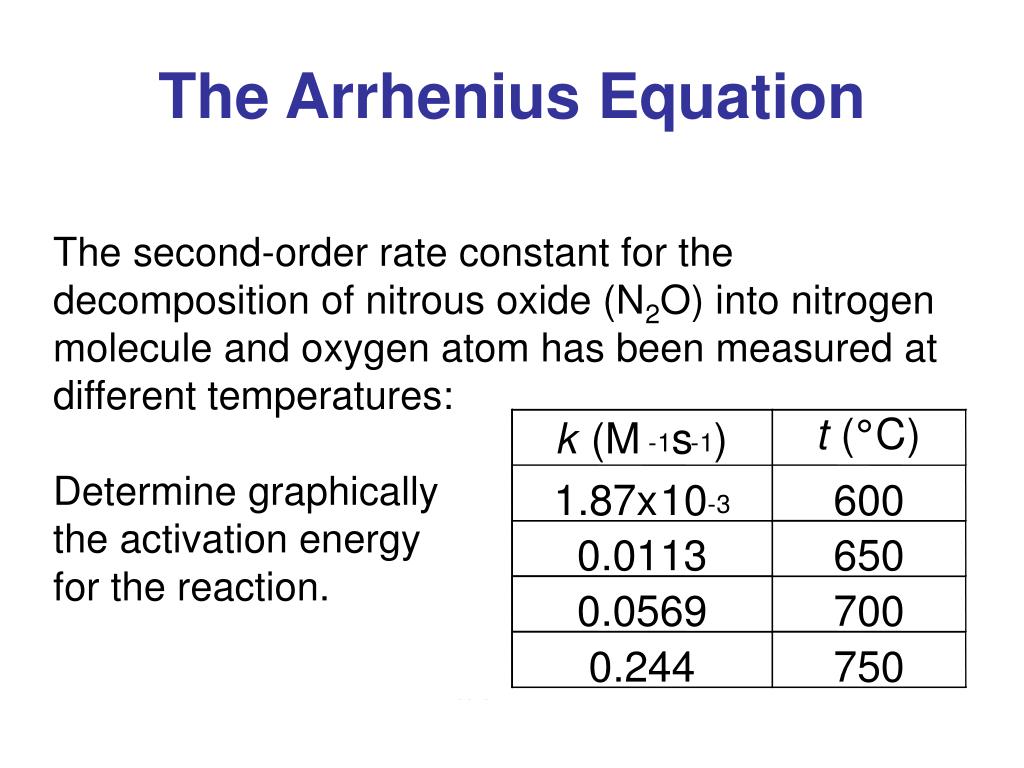
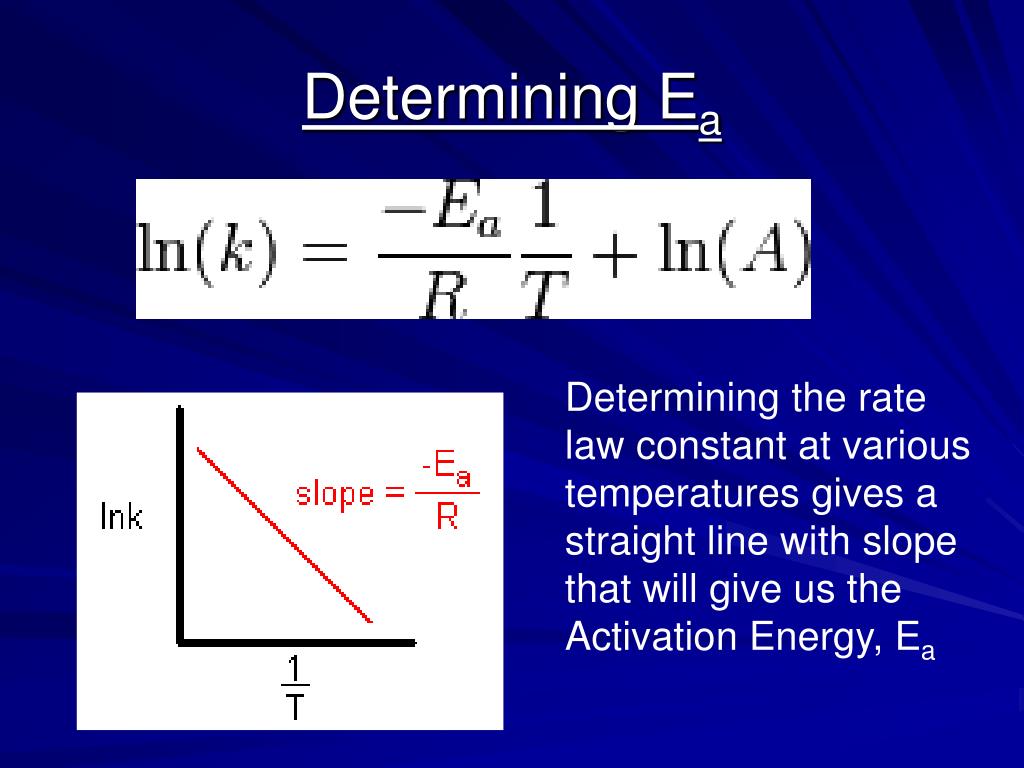
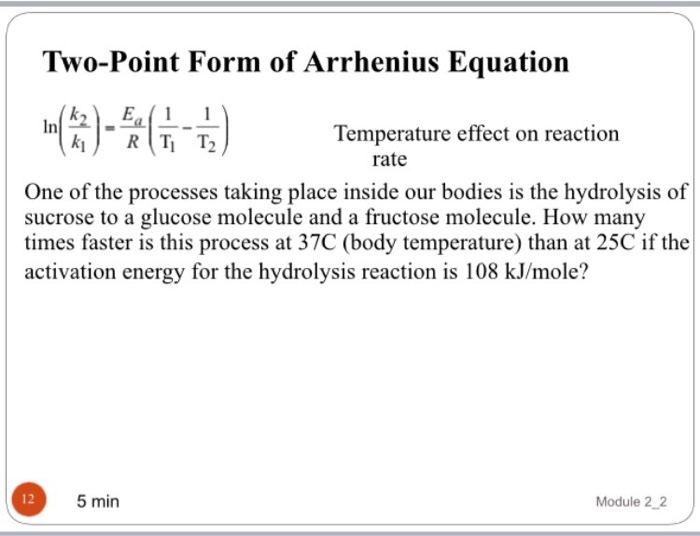
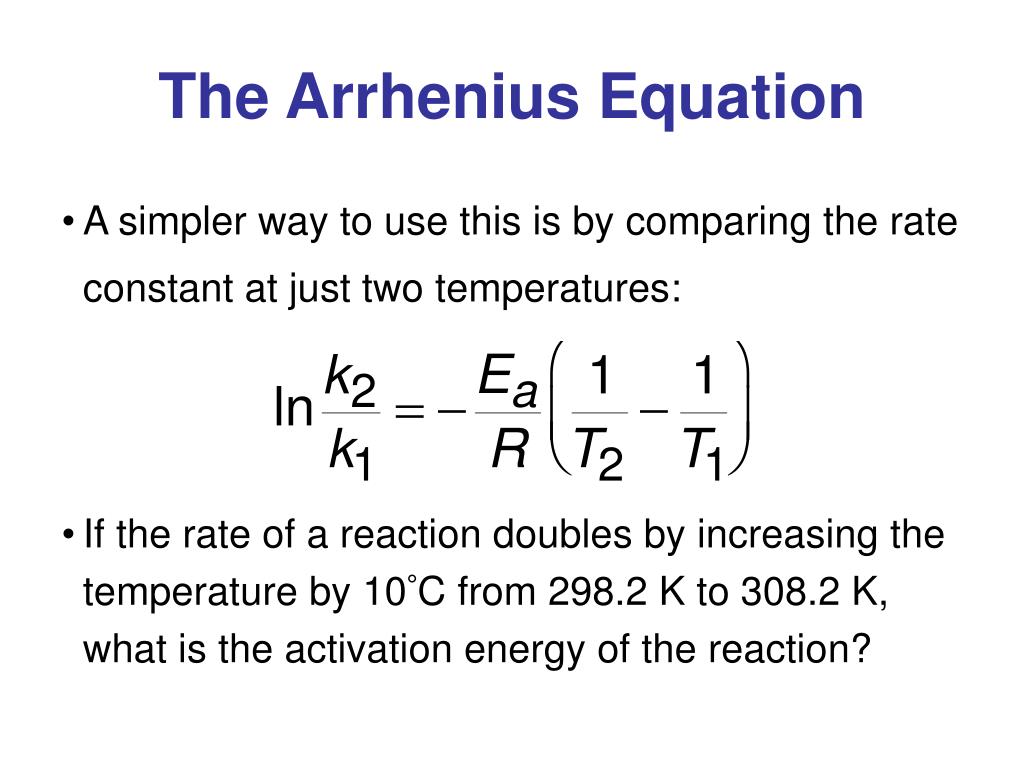
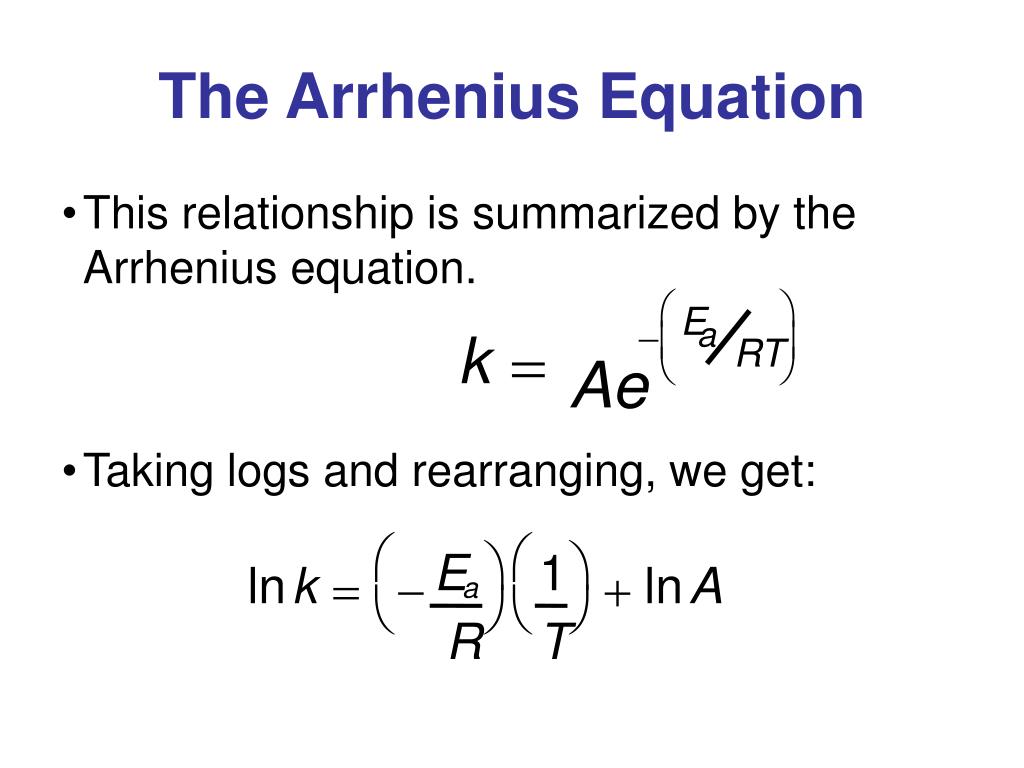
Two Point Form Of The Arrhenius Equation
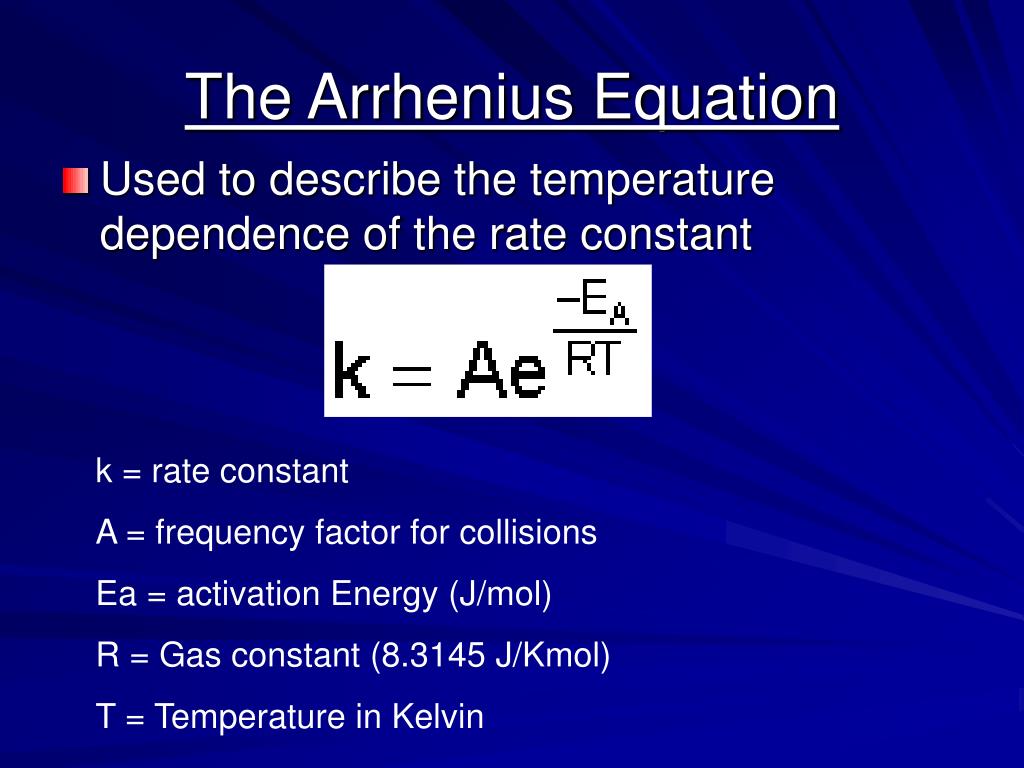
Two Point Form Of The Arrhenius Equation - Find the activation energy for the following reaction: Web the arrhenius equation, k = ae − ea / rt. Using the arrhenius equation to look at how changing temperature and activation energy affects collisions. This variation of the arrhenius equation involves the use of two arrhenius plots constructed on the same graph to determine the activation energy. Lnk = ln(ae − ea / rt) = lna + ln(e − ea / rt) = (− ea r)(1 t) + lna. Web now here when do we use a two point form of the arena's equation? Web the standard form of a linear equation is y = mx + bwhere m is the slop of the line, and b is the y intercept.if you have two points (x1,y1) and (x2,y2), you can get the slope with the following. You should be able to solve for. Slope = − ea r. 2k views 3 years ago chm 152 kinetics. Web the arrhenius equation gives the dependence of the rate constant of a chemical reaction on the absolute temperature as. Web the two point form of the arrhenius equation is a variation of the original equation that can be used to calculate reaction rates at two different temperatures. This variation of the arrhenius equation involves the use of two arrhenius. Web the integrated form of the arrhenius equation is also useful (equation 6.2.3.4.3 6.2.3.4.3 ). You should be able to solve for. Web the standard form of a linear equation is y = mx + bwhere m is the slop of the line, and b is the y intercept.if you have two points (x1,y1) and (x2,y2), you can get the. Can be visualized as the frequency of correctly oriented collisions between reactant particles). Lnk = ln(ae − ea / rt) = lna + ln(e − ea / rt) = (− ea r)(1 t) + lna. Slope = − ea r. 721 views 2 years ago general chemistry 2. Web the two point form of the arrhenius equation is a variation. If the rate constant doubles, for example, so does the rate of the reaction. Web the integrated form of the arrhenius equation is also useful (equation 6.2.3.4.3 6.2.3.4.3 ). You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web how to write different forms of the arrhenius equation. Web the resulting slope allows. At two different temperatures t 1 and t 2, the corresponding values of rate constants k 1 and k 2 are known respectively then, we can write as: This variation of the arrhenius equation involves the use of two arrhenius plots constructed on the same graph to determine the activation energy. If we know the rate constants at two temperatures,. Slope = − ea r. Web the arrhenius equation as it's used in chemistry is often stated according to the formula: Can be visualized as the frequency of correctly oriented collisions between reactant particles). This problem has been solved! Calculate the value for the arrhenius energy of activation, ea, for reaction 1, below. Web the arrhenius equation as it's used in chemistry is often stated according to the formula: Web now here when do we use a two point form of the arena's equation? Web the arrhenius equation can be used to determine the effect of a change of temperature on the rate constant, and consequently on the rate of the reaction. Can. Web use the arrhenius equation: Now the arrhenius equation 2.4 um formula is as. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web the arrhenius equation as it's used in chemistry is often stated according to the formula: If we know the rate constants at two temperatures, then the activation energy can. Web the arrhenius equation can be used to determine the effect of a change of temperature on the rate constant, and consequently on the rate of the reaction. Slope = − ea r. You should be able to solve for. Web how to write different forms of the arrhenius equation. 721 views 2 years ago general chemistry 2. The above equation, shows temperature's effect on multiple rate constants. Slope = − ea r. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. E a is the activation energy of the reaction (usually given in joules per mole or j/mol) If we know the rate constants at two temperatures, then the activation. Using the arrhenius equation to look at how changing temperature and activation energy affects collisions. Web the integrated form of the arrhenius equation is also useful (equation 6.2.3.4.3 6.2.3.4.3 ). Web now here when do we use a two point form of the arena's equation? Web use the arrhenius equation: Web the resulting slope allows us to determine the energy of activation. Web the arrhenius equation as it's used in chemistry is often stated according to the formula: If we know the rate constants at two temperatures, then the activation energy can be found. If the rate constant doubles, for example, so does the rate of the reaction. Calculate the value for the arrhenius energy of activation, ea, for reaction 1, below. 2k views 3 years ago chm 152 kinetics. This variation of the arrhenius equation involves the use of two arrhenius plots constructed on the same graph to determine the activation energy. Web the standard form of a linear equation is y = mx + bwhere m is the slop of the line, and b is the y intercept.if you have two points (x1,y1) and (x2,y2), you can get the slope with the following. Web the arrhenius equation gives the dependence of the rate constant of a chemical reaction on the absolute temperature as. 721 views 2 years ago general chemistry 2. This problem has been solved! Lnk = ln(ae − ea / rt) = lna + ln(e − ea / rt) = (− ea r)(1 t) + lna. The above equation, shows temperature's effect on multiple rate constants. At two different temperatures t 1 and t 2, the corresponding values of rate constants k 1 and k 2 are known respectively then, we can write as: Find the activation energy for the following reaction: A is an exponential factor that is a constant for a given chemical reaction, relating the frequency of collisions of particles.Two point arrhenius equation, Easy derivation, 3 application
Arrhenius Equation 2 data points to solve for k2 or Ea YouTube
arrhenius equation Yahoo Image Search Results Energy activities
PPT The Arrhenius Equation PowerPoint Presentation, free download
PPT The Arrhenius Equation PowerPoint Presentation, free download
Solved TwoPoint Form of Arrhenius Equation In N2.
Chapter13 chemical
PPT The Arrhenius Equation PowerPoint Presentation, free download
PPT The Arrhenius Equation PowerPoint Presentation, free download
PPT The Arrhenius Equation PowerPoint Presentation, free download
Related Post: