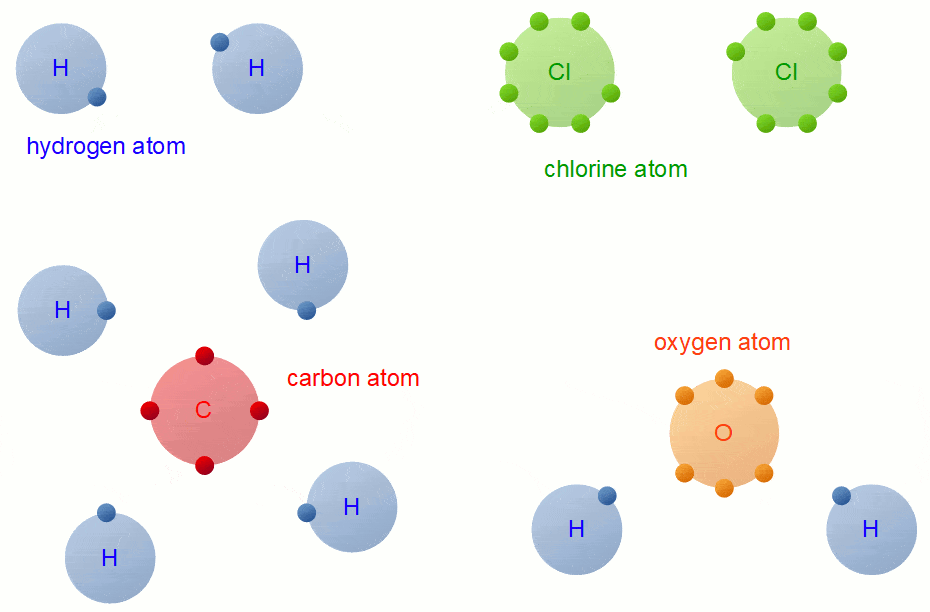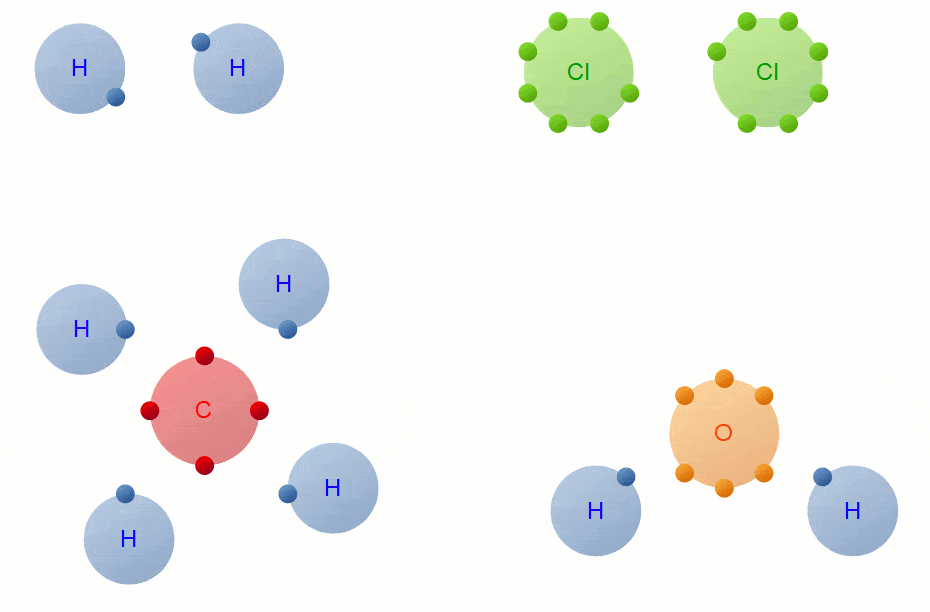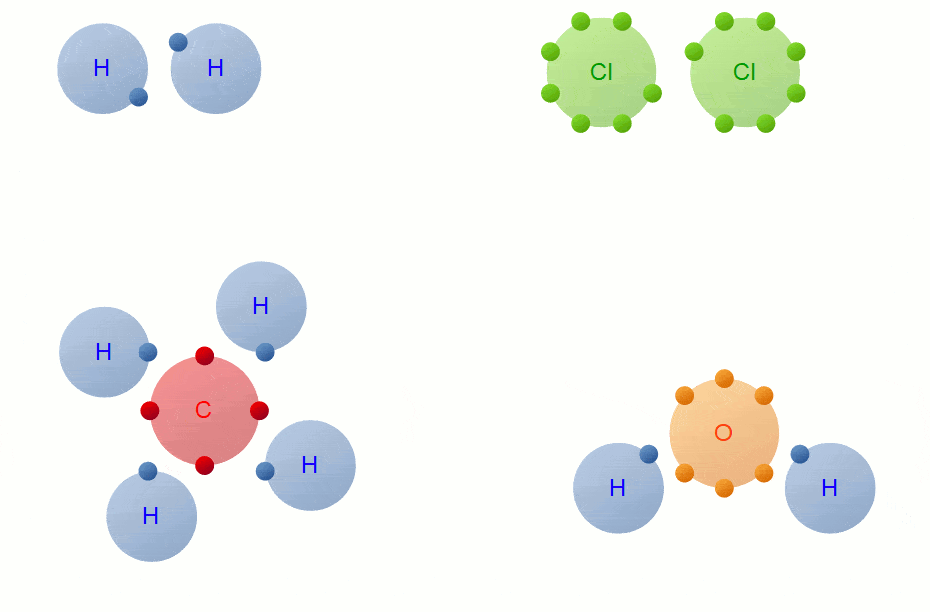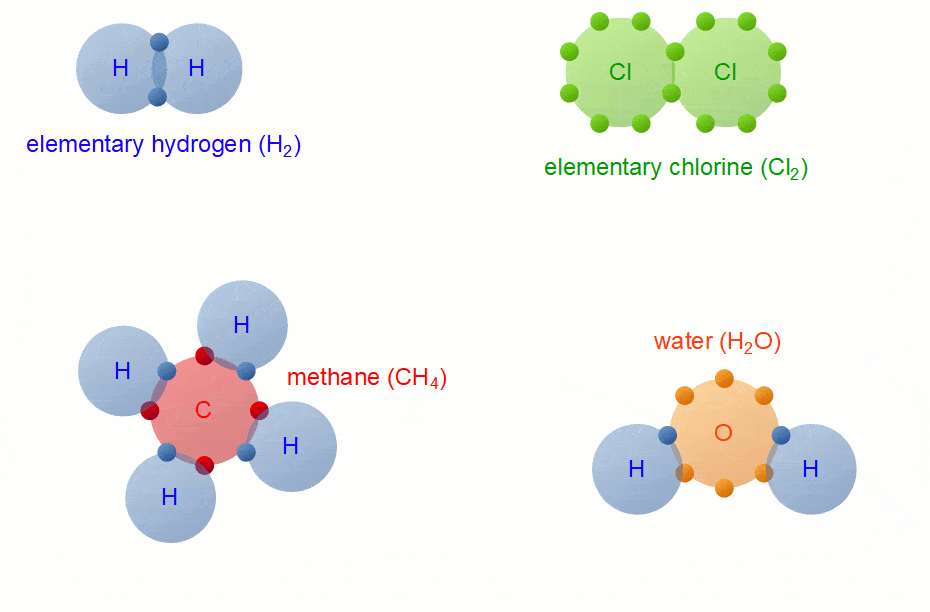
A Carbon Atom Can Form Up To Four Covalent Bonds
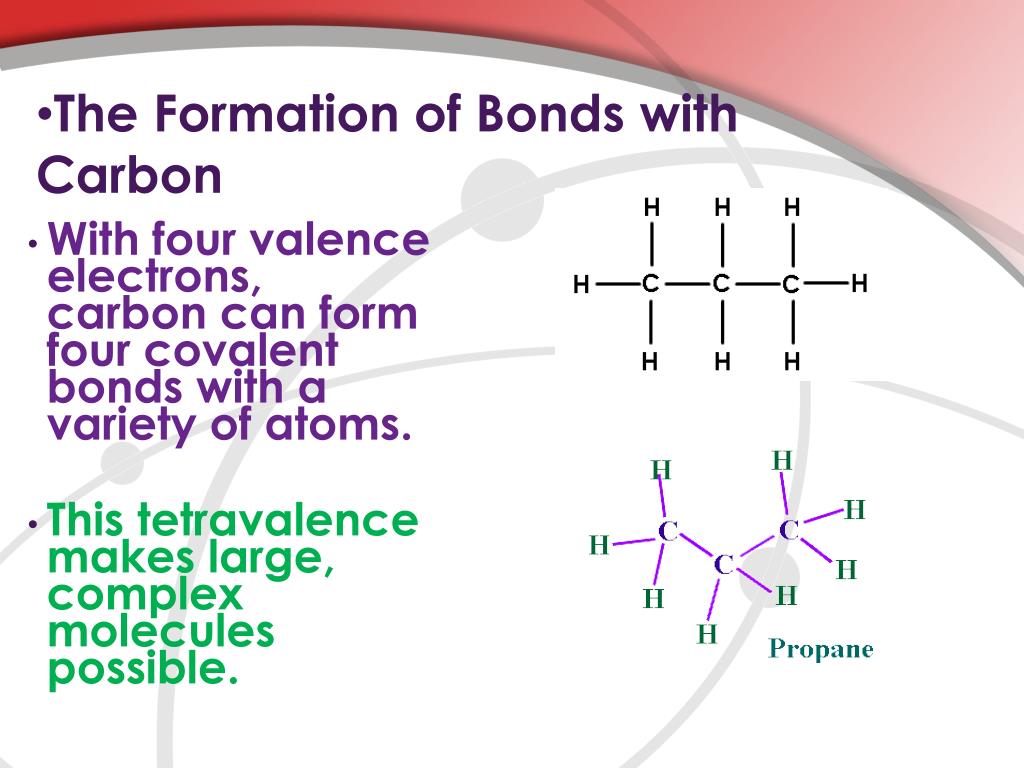
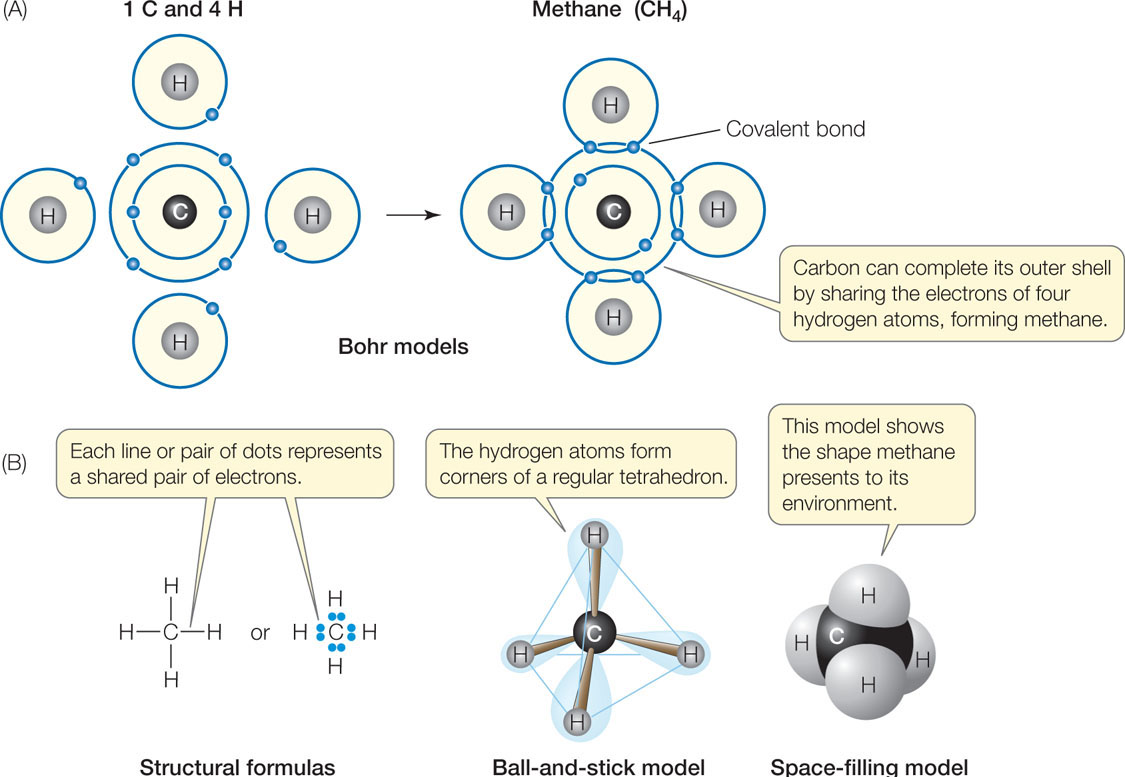
A Carbon Atom Can Form Up To Four Covalent Bonds - A carbon atom has six electrons in its outermost shell. How many valence electrons does a carbon atom have? The bond between a carbon and hydrogen atom is a polar bond. A carbon atom can form up to four covalent bonds. Web the four covalent bonding positions of the carbon atom can give rise to a wide diversity of compounds with many functions, accounting for the importance of carbon in living things. Web therefore, carbon atoms can form up to four covalent bonds with other atoms to satisfy the octet rule. Web moreover, of all the elements in the second row, carbon has the maximum number of outer shell electrons (four) capable of forming covalent bonds. A carbon atom has six electrons in its outermost shell. Carbon contains four electrons in its outer shell. The potential energy of two separate hydrogen atoms (right) decreases as. The carbon atoms form a regular tetrahedral network structure. Web these four electrons can be gained by forming four covalent bonds, as illustrated here for carbon in ch 4 (methane). Web a carbon atom can form up to four covalent bonds. Web therefore, carbon atoms can form up to four covalent bonds with other atoms to satisfy the octet rule.. A carbon atom can form up to four covalent bonds. The bond between a carbon and hydrogen atom is a polar bond. How many valence electrons does a carbon atom have? It has the chemical formula ch 4. Web therefore, carbon atoms can form up to four covalent bonds with other atoms to satisfy the octet rule. Web therefore, carbon atoms can form up to four covalent bonds with other atoms to satisfy the octet rule. With hydrogen, nitrogen, oxygen, and other heteroatoms. For example, in methane (ch 4 ), carbon forms covalent bonds. Web mark the following statements about carbon as true or false. Web therefore, carbon atoms can form up to four covalent bonds. The methane molecule provides an example: Web moreover, of all the elements in the second row, carbon has the maximum number of outer shell electrons (four) capable of forming covalent bonds. The carbon atoms form a regular tetrahedral network structure. Is determined by the distance at which the lowest potential energy is achieved. Web therefore, carbon atoms can form up. It has the chemical formula ch 4. How many valence electrons does a carbon atom have? Web therefore, carbon atoms can form up to four covalent bonds with other atoms to satisfy the octet rule. Web the carbon atom has unique properties that allow it to form covalent bonds to as many as four different atoms, making this versatile element. Web to achieve stability, carbon must find four more electrons to fill its outer shell, giving a total of eight and satisfying the octet rule. A carbon atom has six electrons in its outermost shell. Web the carbon backbone of a molecule is made up of a string of carbon atoms held together with either single or double bonds. Web. Web well, carbon can form up to four covalent bonds. It has the chemical formula ch 4. The methane molecule provides an example: Web moreover, of all the elements in the second row, carbon has the maximum number of outer shell electrons (four) capable of forming covalent bonds. Web a carbon atom can form up to four covalent bonds. It has the chemical formula ch 4. A carbon atom has six electrons in its outermost shell. With hydrogen, nitrogen, oxygen, and other heteroatoms. Group 5a (15) elements such as nitrogen. Web the carbon atom has unique properties that allow it to form covalent bonds to as many as four different atoms, making this versatile element ideal to serve as. Group 5a (15) elements such as nitrogen. The methane molecule provides an example: Web these four electrons can be gained by forming four covalent bonds, as illustrated here for carbon in ch 4 (methane). Web the four covalent bonding positions of the carbon atom can give rise to a wide diversity of compounds with many functions, accounting for the importance. The bond between a carbon and hydrogen atom is a polar bond. The methane molecule provides an example: Web each carbon atom is joined to four other carbon atoms by covalent bonds. Web to achieve stability, carbon must find four more electrons to fill its outer shell, giving a total of eight and satisfying the octet rule. Web therefore, carbon. The bond between a carbon and hydrogen atom is a polar bond. Which of the following types of lipid is the most abundant. It has the chemical formula ch 4. The bond between a carbon and hydrogen atom is a polar bond. Group 5a (15) elements such as nitrogen. A carbon atom can form up to four covalent bonds. For example, in methane (ch 4 ), carbon forms covalent bonds. Therefore, it can form four covalent. Web these four electrons can be gained by forming four covalent bonds, as illustrated here for carbon in ccl 4 (carbon tetrachloride) and silicon in sih 4 (silane). How many valence electrons does a carbon atom have? Web therefore, carbon atoms can form up to four covalent bonds with other atoms to satisfy the octet rule. A carbon atom has six electrons in its outermost shell. The carbon atoms form a regular tetrahedral network structure. The methane molecule provides an example: Web these four electrons can be gained by forming four covalent bonds, as illustrated here for carbon in ch 4 (methane). Web a carbon atom can form up to four covalent bonds. The methane molecule provides an example: Web the carbon backbone of a molecule is made up of a string of carbon atoms held together with either single or double bonds. Web therefore, carbon atoms can form up to four covalent bonds with other atoms to satisfy the octet rule. A carbon atom can form up to four covalent bonds.PPT Biochemistry PowerPoint Presentation, free download ID89333
Four covalent bonds. Carbon has four valence electrons and here a
PPT Carbon PowerPoint Presentation, free download ID1833140
BONDING IN CARBON THE COVALENT BOND YouTube
PPT Unit 1 Biochemistry The Chemistry of Life PowerPoint
The top panel in this figure shows two hydrogen atoms sharing two
Carbon to Carbon Single, Double & Triple Bonds Surfguppy
hillis2e_ch02
Overview of Carbon and Covalent Bonding in Carbon Class 10 Notes EduRev
The 4 Types of Bonds Carbon Can Form Video & Lesson Transcript
Related Post: