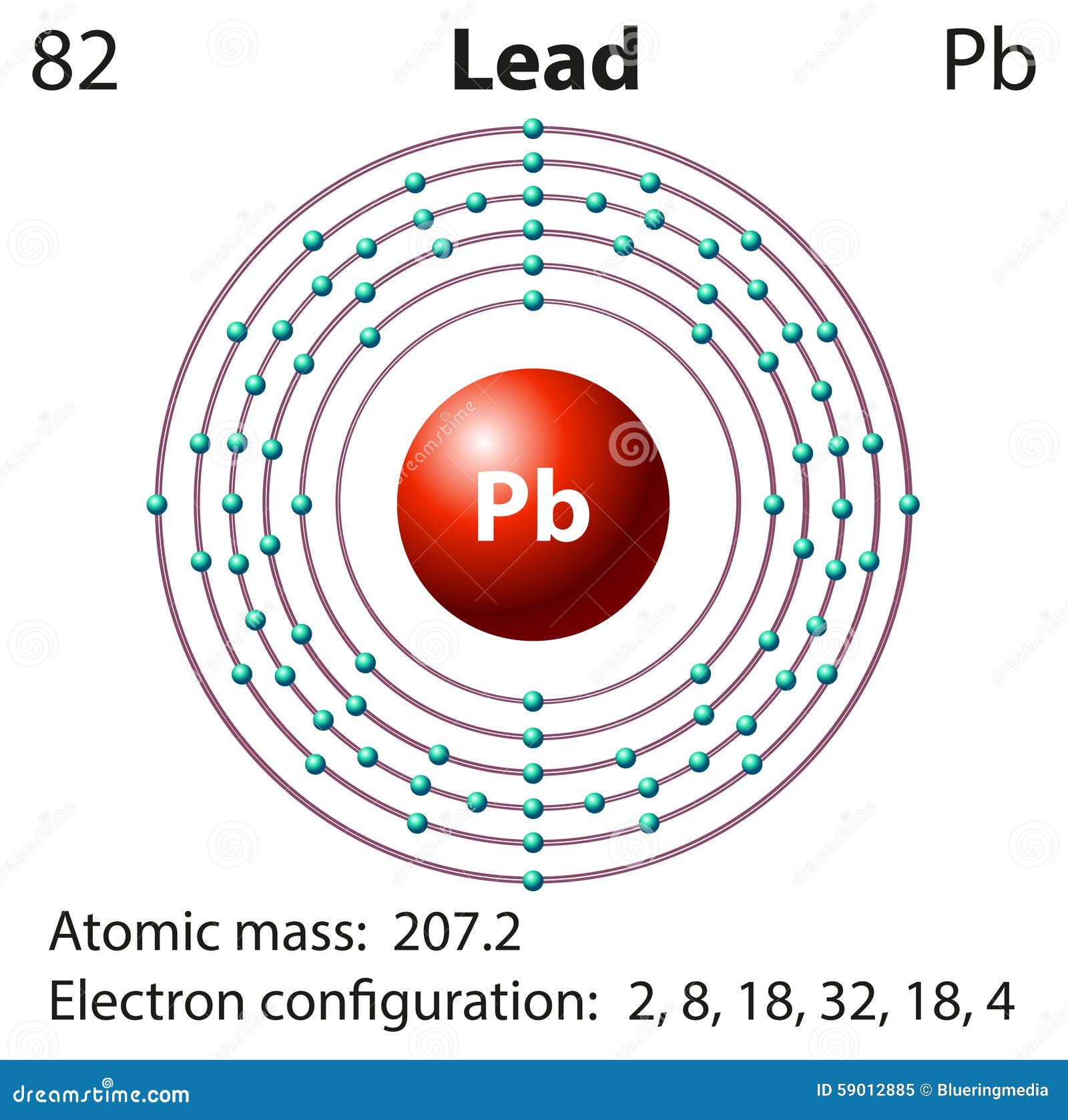
Lead Electron Configuration Long Form
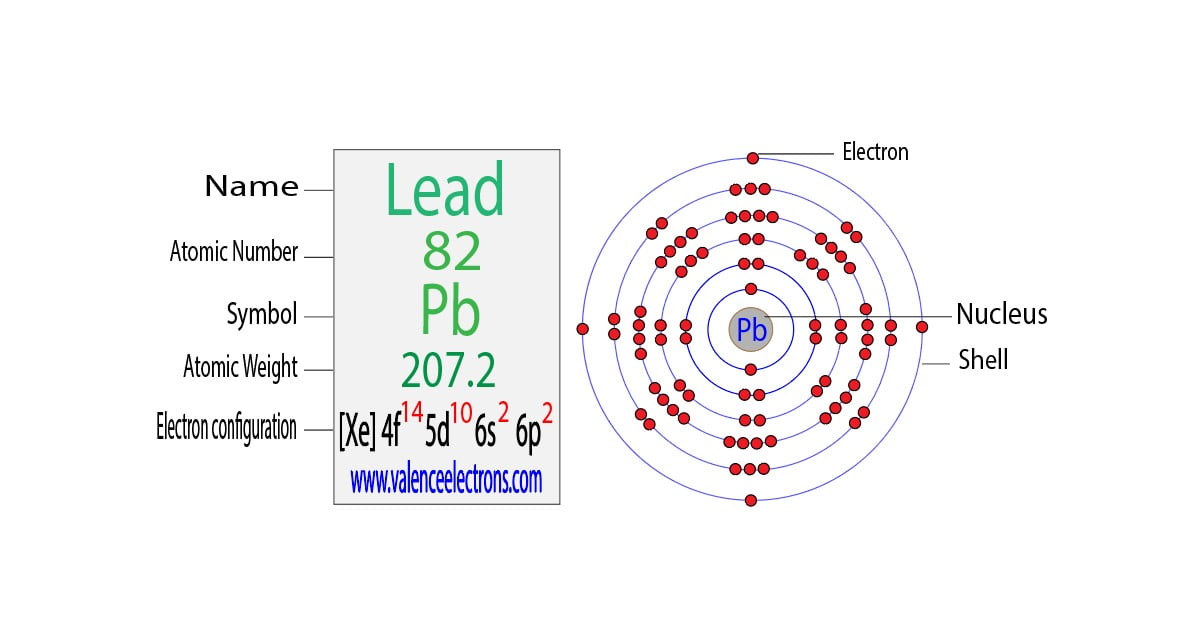
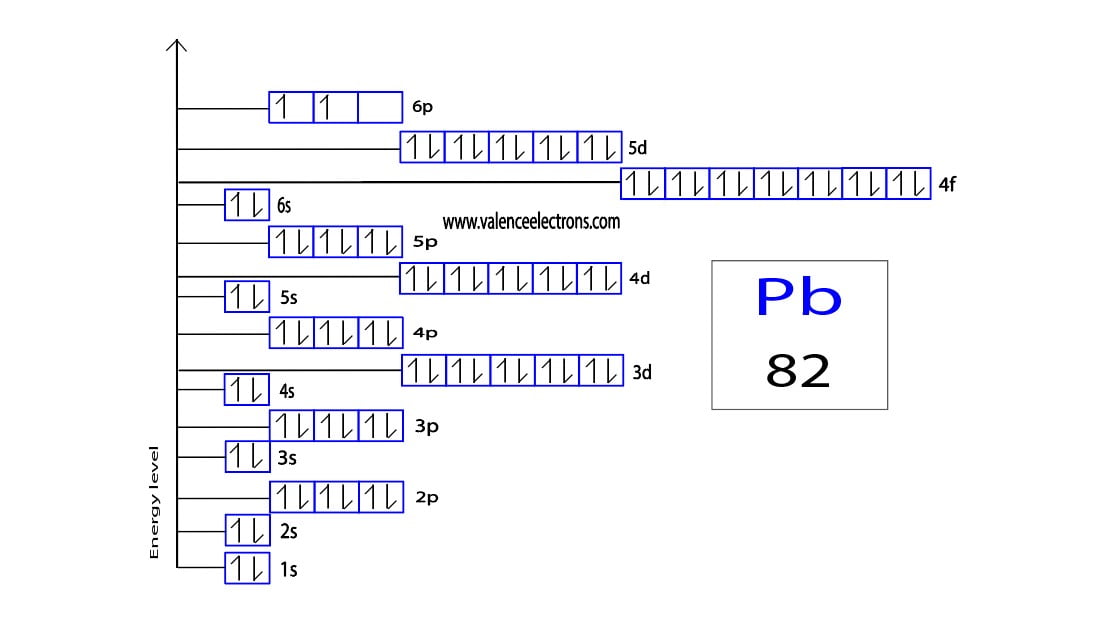
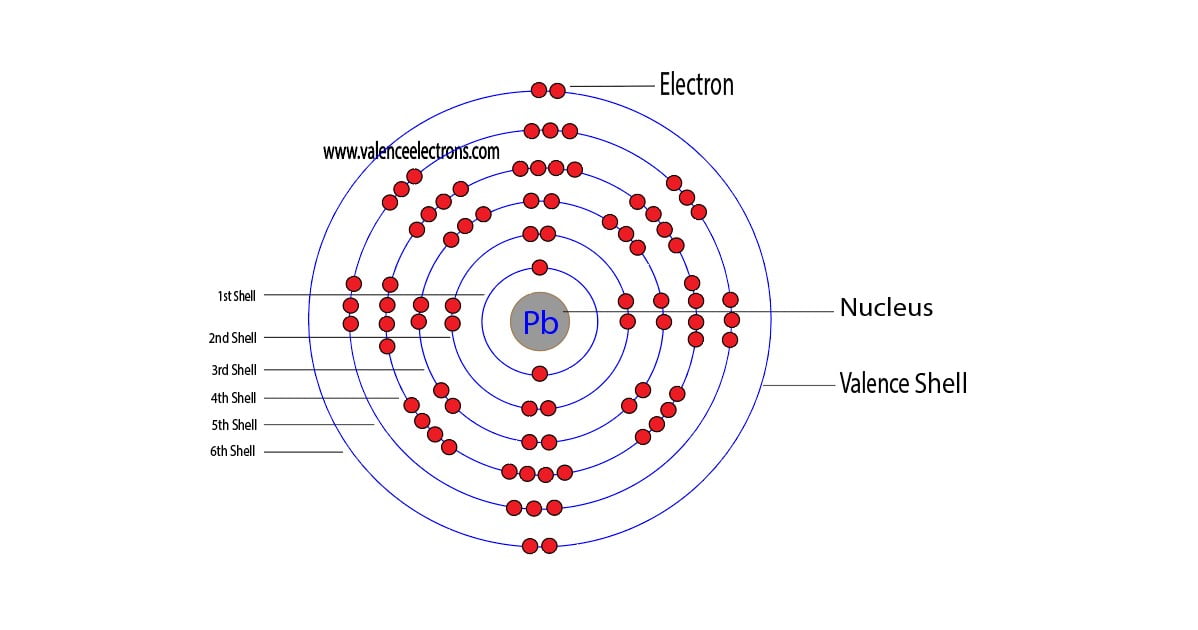
Lead Electron Configuration Long Form - The sum of lead's first and second ionization energies—the total energy required to remove the two 6p electrons—is close to that of tin, lead's upper neighbor in the carbon group. Web the commonly used long form of the periodic table is designed to emphasize electron configurations. Web this page shows the electron configurations of the neutral gaseous atoms in their ground states. Web periodic table element summary lead lead is a chemical element with symbol pb and atomic number 82. Since it is the outermost (valence) electrons which. From wikipedia, the free encyclopedia lead (/lɛd/) is a chemical element in the carbon group with symbol pb (from latin:plumbum) and atomic. Ionization energies generally fall going down a group, as an element's outer electrons become more distant fro… Web by “building up” from hydrogen, this table can be used to determine the electron configuration for any atom on the periodic table. Cross section (thermal neutron capture) σ a /barns: Web when the lead is freshly cut, its surface is bluish grey. Electron configuration the periodic table is a tabular display of the chemical. Web the electron configuration of iridium is much longer than aluminum. Web in the case of lead the abbreviated electron configuration is [xe] 4f14 5d10 6s2 6p2. Web atomic structure of lead. For each atom the subshells are given first in concise form, then with all. Web atomic structure of lead. Web periodic table element summary lead lead is a chemical element with symbol pb and atomic number 82. Web electron configuration of lead is [hg] 6p2. Electron configuration the periodic table is a tabular display of the chemical. We describe an electron configuration with a symbol that contains three. >> back to key information about the element. From wikipedia, the free encyclopedia lead (/lɛd/) is a chemical element in the carbon group with symbol pb (from latin:plumbum) and atomic. A lead atom has 82 electrons, arranged in an electron configuration of [xe]4f 5d 6s 6p. Web atomic structure of lead. Web when the lead is freshly cut, its surface. Web atomic structure of lead. Web this page shows the electron configurations of the neutral gaseous atoms in their ground states. Electron configuration the periodic table is a tabular display of the chemical. Since it is the outermost (valence) electrons which. >> back to key information about the element. Web periodic table element summary lead lead is a chemical element with symbol pb and atomic number 82. Web by “building up” from hydrogen, this table can be used to determine the electron configuration for any atom on the periodic table. Web in the case of lead the abbreviated electron configuration is [xe] 4f14 5d10 6s2 6p2. Ionization energies generally. Web when the lead is freshly cut, its surface is bluish grey. We describe an electron configuration with a symbol that contains three. Since it is the outermost (valence) electrons which. Web the electron configuration of iridium is much longer than aluminum. Web atomic structure of lead. We describe an electron configuration with a symbol that contains three. Since it is the outermost (valence) electrons which. For each atom the subshells are given first in concise form, then with all. Web when the lead is freshly cut, its surface is bluish grey. Web the commonly used long form of the periodic table is designed to emphasize electron. Web when the lead is freshly cut, its surface is bluish grey. We describe an electron configuration with a symbol that contains three. A vertical column in the periodic table. Web periodic table element summary lead lead is a chemical element with symbol pb and atomic number 82. For each atom the subshells are given first in concise form, then. A poor conductor of electricity, lead is. From wikipedia, the free encyclopedia lead (/lɛd/) is a chemical element in the carbon group with symbol pb (from latin:plumbum) and atomic. Nevertheless, check the complete configuration and other interesting facts. Since it is the outermost (valence) electrons which. Cross section (thermal neutron capture) σ a /barns: Web by “building up” from hydrogen, this table can be used to determine the electron configuration for any atom on the periodic table. Web when the lead is freshly cut, its surface is bluish grey. Web in the case of lead the abbreviated electron configuration is [xe] 4f14 5d10 6s2 6p2. But if it is kept open in air, then. Web electron configuration of lead is [hg] 6p2. From wikipedia, the free encyclopedia lead (/lɛd/) is a chemical element in the carbon group with symbol pb (from latin:plumbum) and atomic. Web the arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. The sum of lead's first and second ionization energies—the total energy required to remove the two 6p electrons—is close to that of tin, lead's upper neighbor in the carbon group. Although drawing out each orbital may prove to be helpful in determining unpaired electrons, it is very time. Ionization energies generally fall going down a group, as an element's outer electrons become more distant fro… We describe an electron configuration with a symbol that contains three. Web in the case of lead the abbreviated electron configuration is [xe] 4f14 5d10 6s2 6p2. Web the commonly used long form of the periodic table is designed to emphasize electron configurations. Nevertheless, check the complete configuration and other interesting facts. Cross section (thermal neutron capture) σ a /barns: For each atom the subshells are given first in concise form, then with all. Since it is the outermost (valence) electrons which. A lead atom has 82 electrons, arranged in an electron configuration of [xe]4f 5d 6s 6p. But if it is kept open in air, then it reacts with oxygen and starts tarnishing (which forms a grey oxide layer on it). A vertical column in the periodic table. Web this page shows the electron configurations of the neutral gaseous atoms in their ground states. A poor conductor of electricity, lead is. >> back to key information about the element. Members of a group typically have similar properties and electron configurations in their outer shell.Electron Configuration for Android APK Download
Lead, atomic structure Stock Image C018/3763 Science Photo Library
Electron Configuration for Pb, Pb2+, and Pb4+ (Lead and Lead Ions
List of Electron Configurations of Elements
Lead(Pb) electron configuration and orbital diagram (2023)
Lead(Pb) electron configuration and orbital diagram (2023)
Atom Diagrams Electron Configurations of the Elements
How To Find an Valence Lead Electron Configuration (Pb)
Diagram Representation of the Element Lead Stock Vector Illustration
Complete Electron Configuration for Lead (Pb, Pb2+, Pb4+)
Related Post:




:max_bytes(150000):strip_icc()/Lead-58b601095f9b5860464ba934.jpg)