Will Argon Tend To Form Bonds With Other Elements
Will Argon Tend To Form Bonds With Other Elements - A clue comes by considering the noble gas elements,. Web what causes atoms to make a chemical bond with other atoms, rather than remaining as individual atoms? Just answer the questions and get along with me. Web covalent bonds form when electrons are shared between atoms and are attracted by the nuclei of both atoms. Web this makes it a noble gas, which are generally unreactive and do not tend to form bonds with other elements. The atomic number of argon is 18. Will argon tend to form bonds with other elements? Low a potassium atom is most likely to form __________ bonds with other atoms. A clue comes by considering the noble gas elements, the rightmost. Argon is one of the noble gases (group 18) and thus does not normally combine with other elements. No, since argon has inert gas configuration and has a stable configuration, it does not form any bonds. Web we would like to show you a description here but the site won’t allow us. Web this is also not possible with argon because it already has a full outer shell of electrons and does not need to share any. Web. Web the atomic number of argon is 18. This full valence shell makes argon very stable and extremely resistant to bonding with other elements. Web argon and the other noble gases in the far right group (column) of the periodic table have a full outer shell of electrons and cannot form bonds with other. No, since argon has inert gas. Web what causes atoms to make a chemical bond with other atoms, rather than remaining as individual atoms? Web if argon doesn’t form ions to bond with other elements can it even be ionic? Low a potassium atom is most likely to form __________ bonds with other atoms. Will argon tend to form bonds with other elements? Web what causes. Will argon tend to form bonds with other elements? Ionic bonds, covalent bonds, and metallic bonds. We got some fill in the blank answers here today with a kind of paragraph. Elements in other groups have. Web as we will see below, the periodic table organizes elements in a way that reflects their number and pattern of electrons, which makes. Web what causes atoms to make a chemical bond with other atoms, rather than remaining as individual atoms? Argon is one of the noble gases (group 18) and thus does not normally combine with other elements. However, compounds of the heavier noble gases have since been synthesized. This full valence shell makes argon very stable and extremely resistant to bonding. Web the atomic number of argon is 18. I would like to thank the. Elements in other groups have. We got some fill in the blank answers here today with a kind of paragraph. No, since argon has inert gas configuration and has a stable configuration, it does not form any bonds. However, compounds of the heavier noble gases have since been synthesized. A clue comes by considering the noble gas elements, the rightmost. Web will argon tend to form bonds with other elements? Web argon's name comes from the greek word argos meaning lazy and indeed for more than a hundred years after its discovery chemists were unable to get it. Argon's complete octet of electrons indicates full s and p subshells. Web covalent bonds form when electrons are shared between atoms and are attracted by the nuclei of both atoms. Therefore, based on its electron configuration and position on the. Low a potassium atom is most likely to form __________ bonds with other atoms. Web the atomic number of argon. Web this is also not possible with argon because it already has a full outer shell of electrons and does not need to share any. Web as we will see below, the periodic table organizes elements in a way that reflects their number and pattern of electrons, which makes it useful for predicting the reactivity of an. Therefore, based on. Web what causes atoms to make a chemical bond with other atoms, rather than remaining as individual atoms? Elements in other groups have. Web this makes it a noble gas, which are generally unreactive and do not tend to form bonds with other elements. Will argon tend to form bonds with other elements? Argon's complete octet of electrons indicates full. Web group 18 elements (helium, neon, and argon) have a full outer, or valence, shell. Web 1 a potassium atom has __________ electronegativity. Web the number of electrons in the outermost shell of a particular atom determines its reactivity, or tendency to form chemical bonds with other atoms. The atomic number of argon is 18. In pure covalent bonds, the electrons are shared equally. Web the atomic number of argon is 18. We got some fill in the blank answers here today with a kind of paragraph. Web this is also not possible with argon because it already has a full outer shell of electrons and does not need to share any. Therefore, based on its electron configuration and position on the. A clue comes by considering the noble gas elements,. Low a potassium atom is most likely to form __________ bonds with other atoms. Web argon and the other noble gases in the far right group (column) of the periodic table have a full outer shell of electrons and cannot form bonds with other. Web we would like to show you a description here but the site won’t allow us. Web although there are no sharply defined boundaries, chemical bonds are typically classified into three main types: I would like to thank the. Web if argon doesn’t form ions to bond with other elements can it even be ionic? Just answer the questions and get along with me. No, since argon has inert gas configuration and has a stable configuration, it does not form any bonds. Will argon tend to form bonds with other elements? Elements in other groups have.The Periodic Table and Bonding Mrs. Sanborn's Site
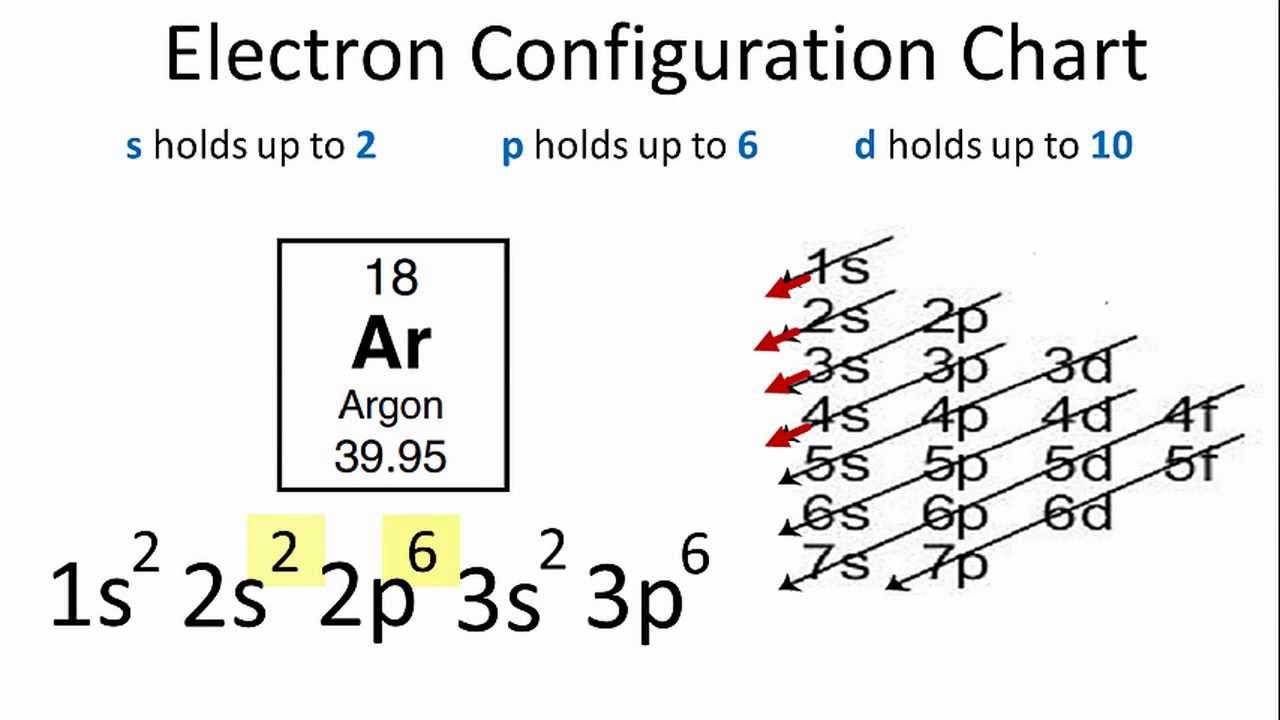
Argon Electron Configuration (Ar) with Orbital Diagram
Forms of Binding in Crystals Overall Science
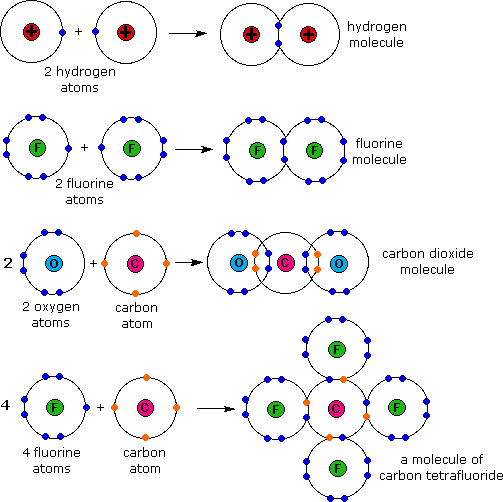
Reading Covalent Bonds Biology I
LabXchange
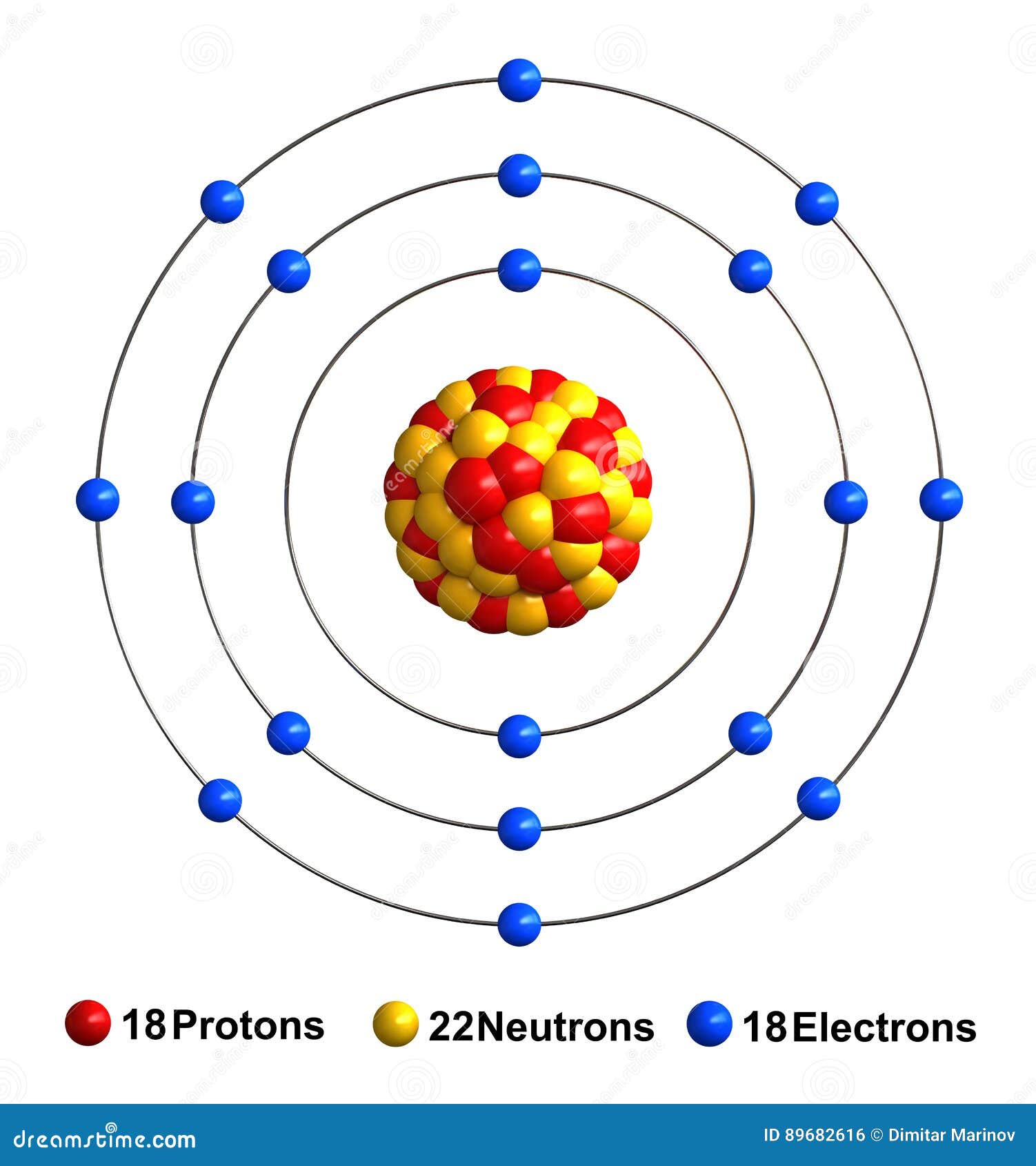
Bohr Diagram For Argon General Wiring Diagram
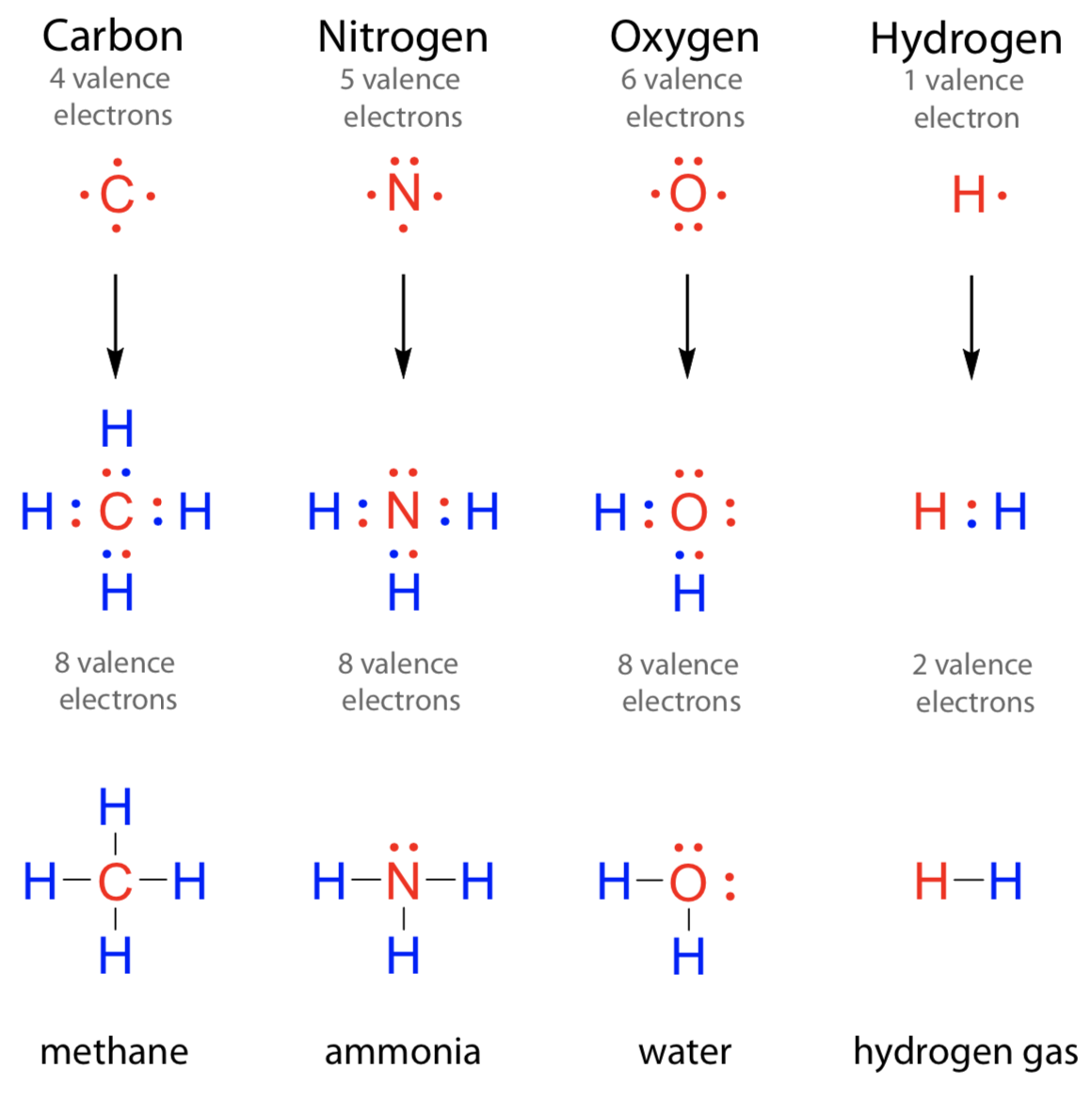
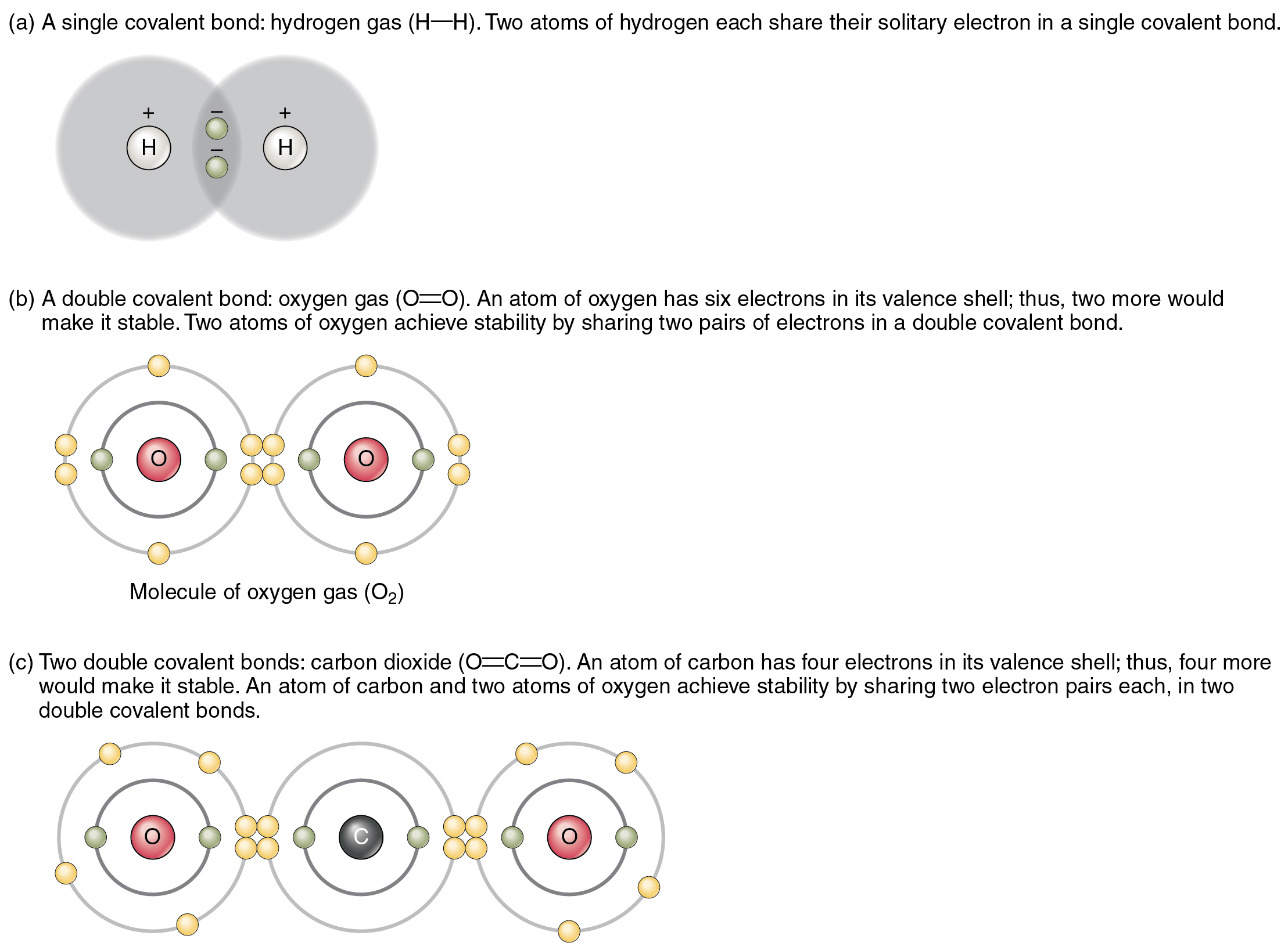
Chemical Bonds · Anatomy and Physiology
Argon Ar (Element 18) of Periodic Table Elements FlashCards
Blog
How to find Valency? What are valence electrons? Teachoo
Related Post: