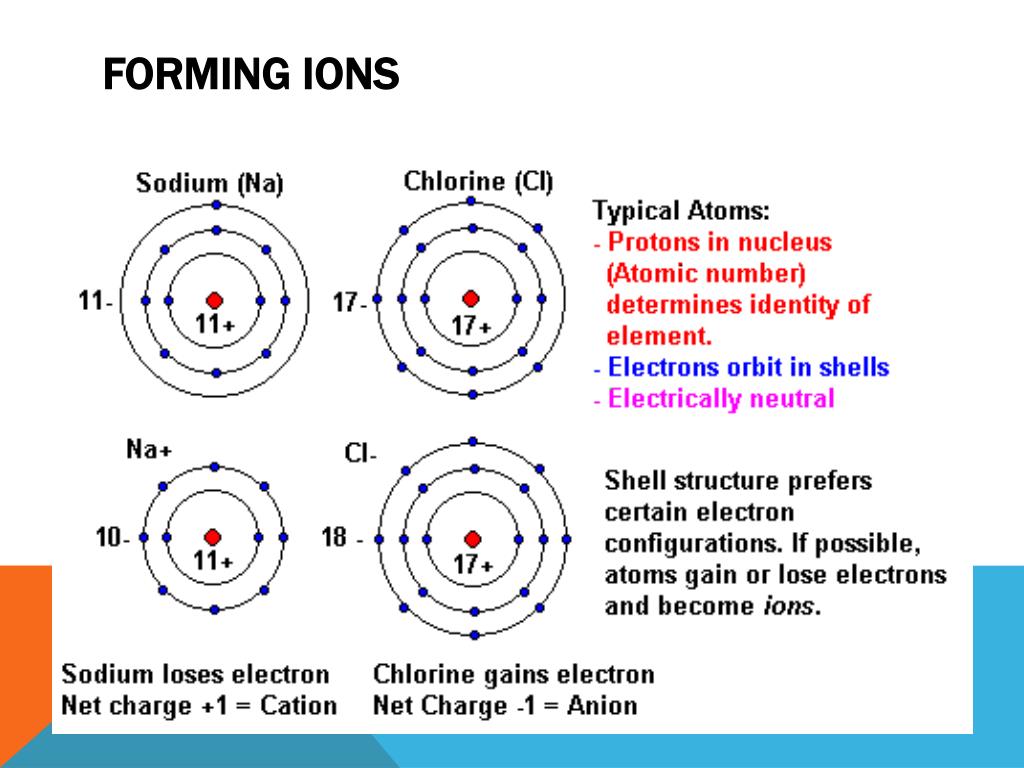
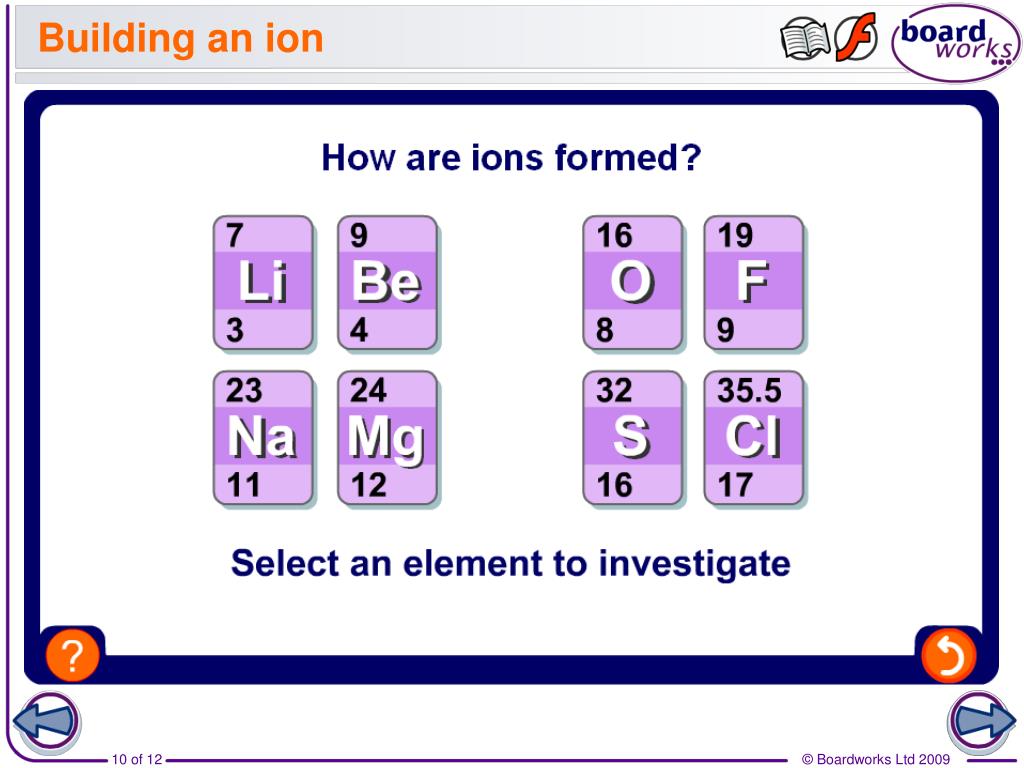
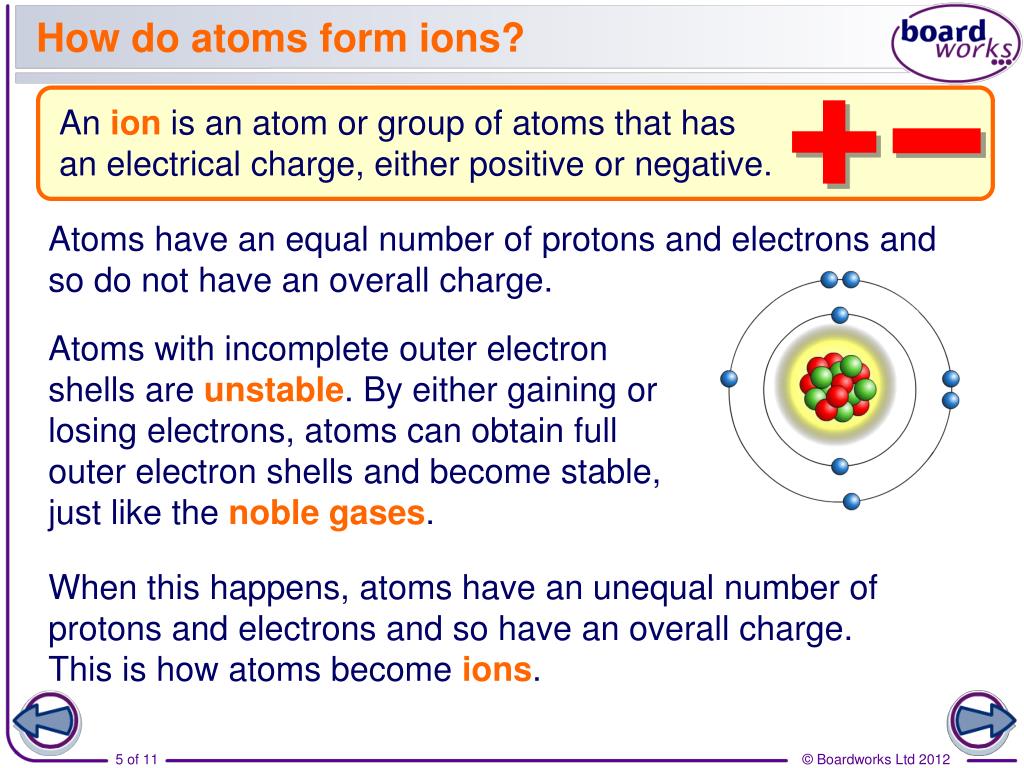
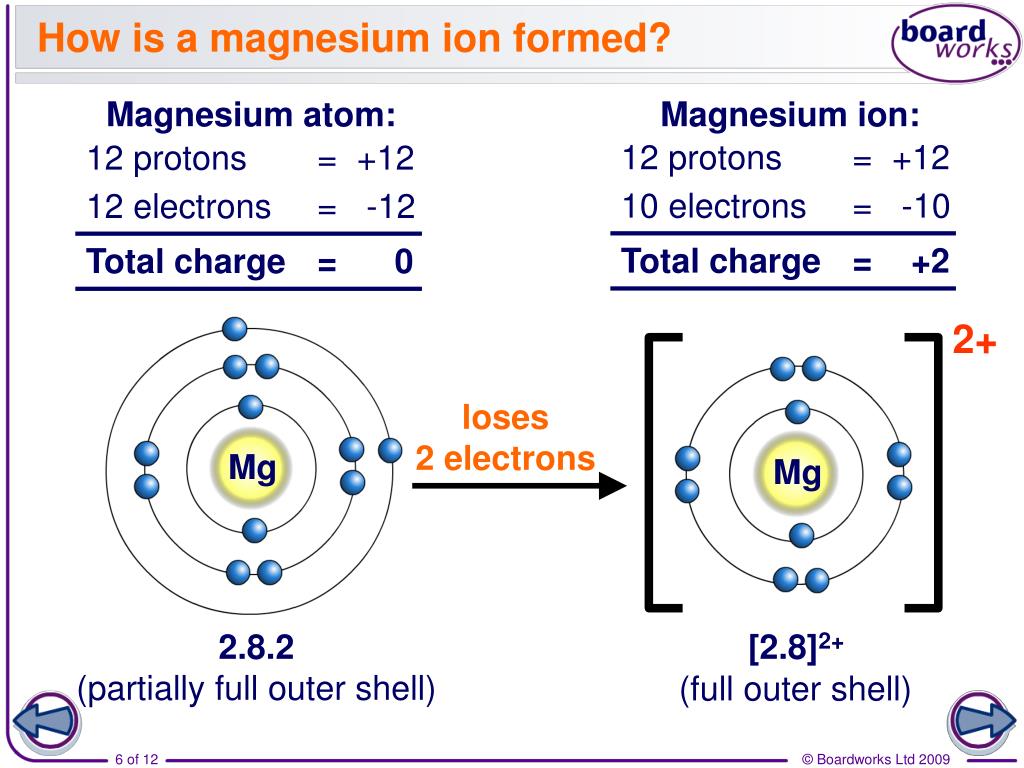
Why Do Elements Form Ions
Why Do Elements Form Ions - Elements form ions to gain stability by attaining noble gas configuration.they lss or. Sodum is a cation (lost an electron and became positive) and chlorine is an anion (gained an electron and. Ions are of two types: The ions have the electronic structure of a noble gas (group 0 element), with a full outer shell. Web every element in the second column forms a cation with charge 2+. For example, fluorine has seven valence electrons,. An ion is a positively or negatively. Web ion, any atom or group of atoms that bears one or more positive or negative electrical charges. Compounds formed from positive and. Web why do most of elements form ions while entering into chemical reactions? Web ions form when two or more elements need to combine together to form a new one. Web why do most of elements form ions while entering into chemical reactions? Web two types of ions can be created due to the gain and loss of electrons, they are called cations and anions. Cations or positively charged ions, and anions or. Change the number of neutrons in an atom and it becomes an. Atoms of group 17 gain one electron and form anions with a 1−. Atoms are made up of protons, neutrons and electrons. Web when atoms of nonmetal elements form ions, they generally gain enough electrons to give them the same number of electrons as an atom of the. Positively charged ions are called cations; Sodum is a cation (lost an electron and became positive) and chlorine is an anion (gained an electron and. Wait a moment and try again. Molecules are the simplest unit of a covalent compound, and molecules can be represented in many. A proton never moves from one atom to another. Web when atoms of nonmetal elements form ions, they generally gain enough electrons to give them the same number of electrons as an atom of the next noble gas in the periodic table. Sodum is a cation (lost an electron and became positive) and chlorine is an anion (gained an electron and. Ions form when an atom gains or loses. Web ion, any atom or group of atoms that bears one or more positive or negative electrical charges. The ions have the electronic structure of a noble gas (group 0 element), with a full outer shell. Elements form ions to gain stability by attaining noble gas configuration.they lss or. Web an ion is formed when the atom gains or loses. Web for example, when chlorine takes an electron from sodium, and sodium gives that electron to chlorine, they become ions and form nacl. Atoms of group 17 gain one electron and form anions with a 1−. Web remember that ions are formed only when electrons move from one atom to another; Web jan 7, 2018. Compounds can be classified as. Web the ions formed are negative, because they have more electrons than protons. Web every element in the second column forms a cation with charge 2+. Web for example, when chlorine takes an electron from sodium, and sodium gives that electron to chlorine, they become ions and form nacl. Web when atoms of nonmetal elements form ions, they generally gain. Web jan 7, 2018. Web when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. Elements form ions to gain stability by attaining noble gas configuration.they lss or. Molecules are the simplest unit of a covalent compound, and molecules can be represented in many. Ions form when an atom gains or. Atoms are made up of protons, neutrons and electrons. Web when atoms of nonmetal elements form ions, they generally gain enough electrons to give them the same number of electrons as an atom of the next noble gas in the periodic table. Atoms of group 17 gain one electron and form anions with a 1−. Web the ions formed are. Web every element in the second column forms a cation with charge 2+. Change the number of neutrons in an atom and it becomes an. Web why do most of elements form ions while entering into chemical reactions? Atoms are made up of protons, neutrons and electrons. Wait a moment and try again. Cations or positively charged ions, and anions or negatively charged ions. Web ion, any atom or group of atoms that bears one or more positive or negative electrical charges. Web when atoms of nonmetal elements form ions, they generally gain enough electrons to give them the same number of electrons as an atom of the next noble gas in the periodic. Ions are of two types: Web an ion is formed when the atom gains or loses an electron. Compounds can be classified as ionic or covalent. Change the number of neutrons in an atom and it becomes an. The ions have the electronic structure of a noble gas (group 0 element), with a full outer shell. Elements form ions to gain stability by attaining noble gas configuration.they lss or. For example, fluorine has seven valence electrons,. Atoms of group 17 gain one electron and form anions with a 1−. Wait a moment and try again. Web remember that ions are formed only when electrons move from one atom to another; Compounds formed from positive and. Positively charged ions are called cations; Web the ions formed are negative, because they have more electrons than protons. Web jan 7, 2018. Web two types of ions can be created due to the gain and loss of electrons, they are called cations and anions. Web the transfer of an electron creates ions — cations (positive charge) and anions (negative charge) — and opposite charges attract each other. Web when atoms of nonmetal elements form ions, they generally gain enough electrons to give them the same number of electrons as an atom of the next noble gas in the periodic table.Ionic Bond Definition, Types, Properties & Examples
How Do Ions Form Ionic Bonds
PPT Chapter 2 A quick review of Chemistry PowerPoint Presentation
PPT 1 Name the ions formed by these elements and classify them as
PPT How do atoms form ions? PowerPoint Presentation, free download
PPT Ions PowerPoint Presentation, free download ID6738771
PPT How do atoms form ions? PowerPoint Presentation, free download
Ions
PPT How do atoms form ions? PowerPoint Presentation, free download
PPT How do atoms form ions? PowerPoint Presentation, free download
Related Post: