Why Are Halogens And Alkali Metals Likely To Form Ions
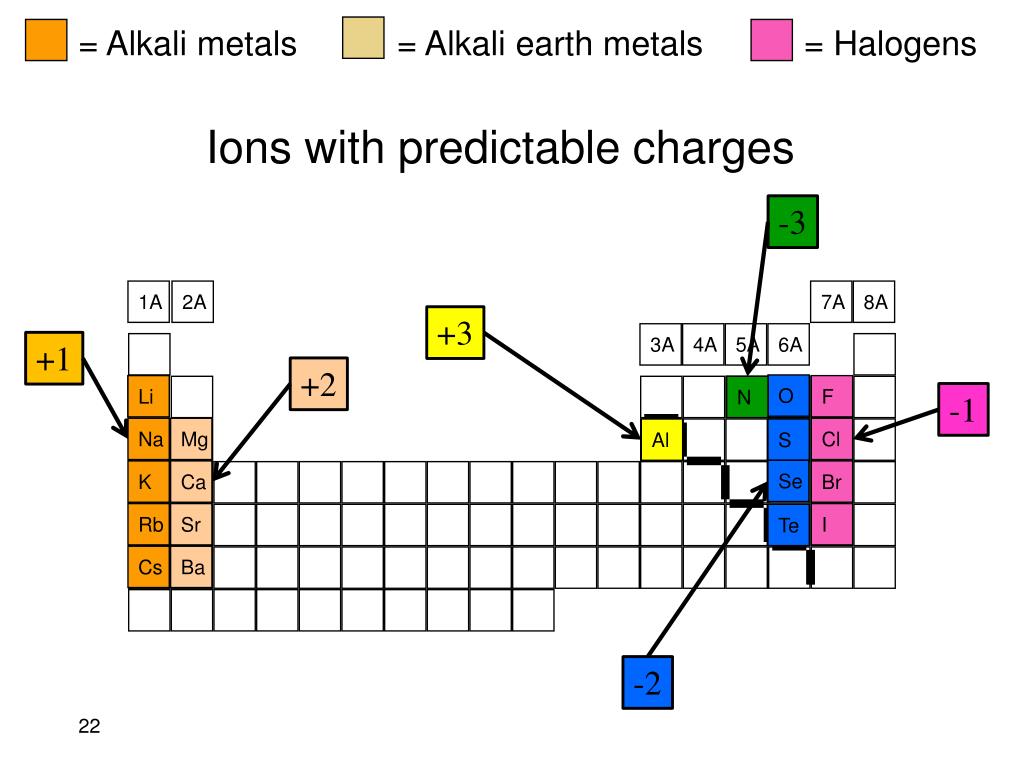
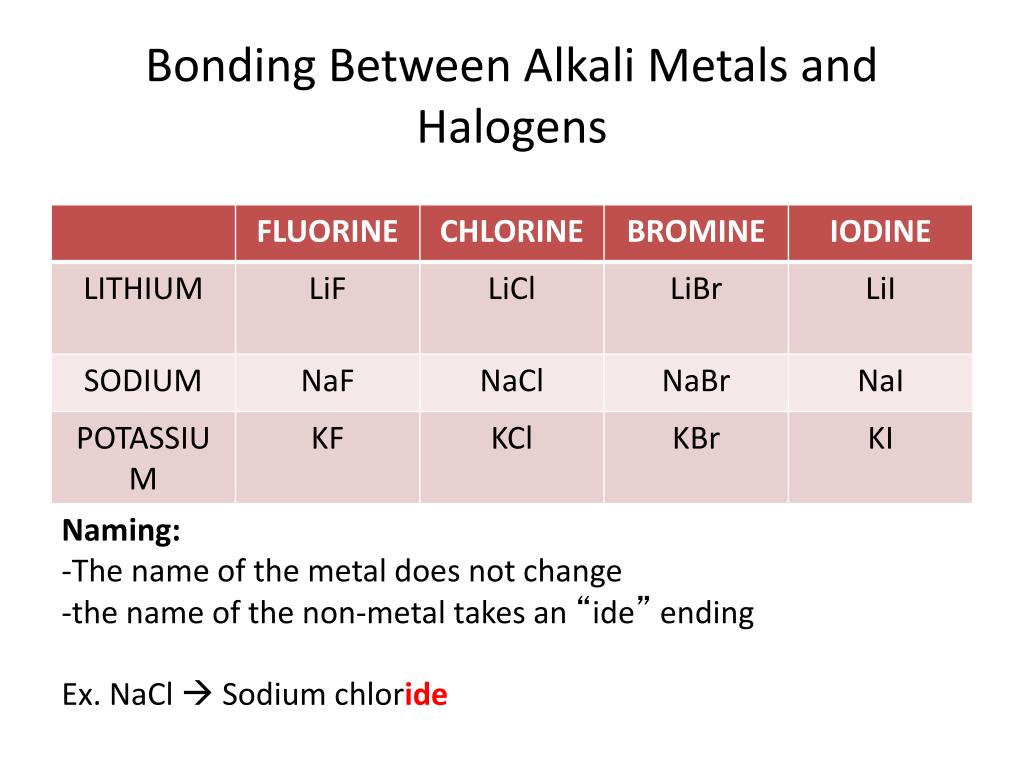
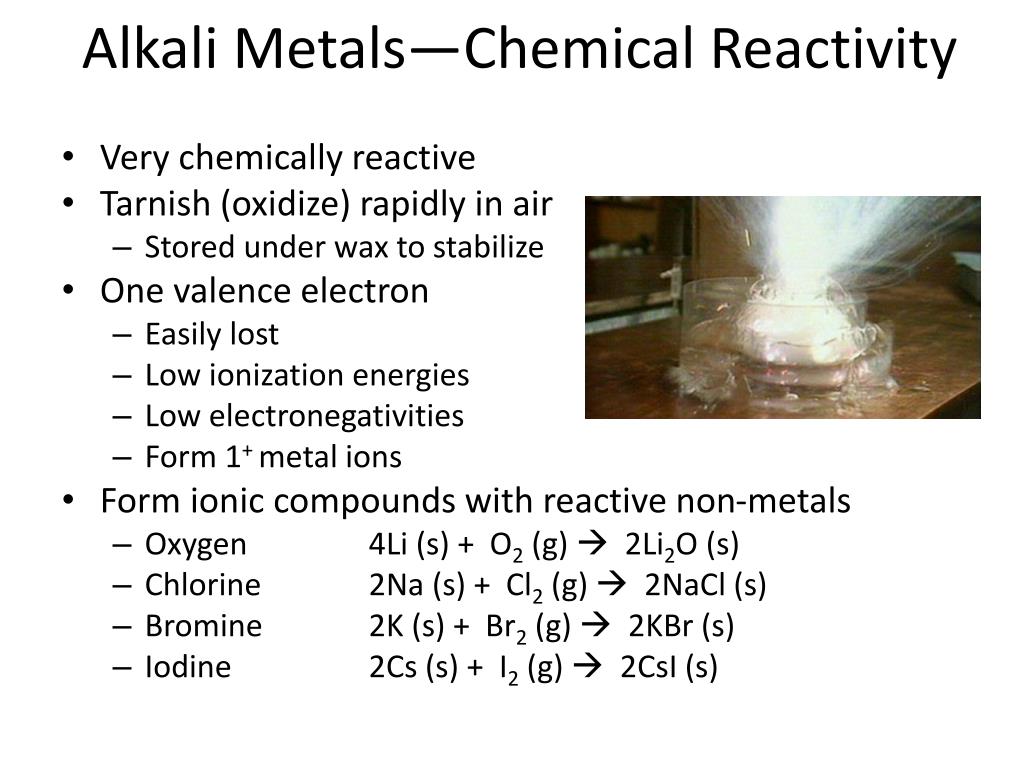
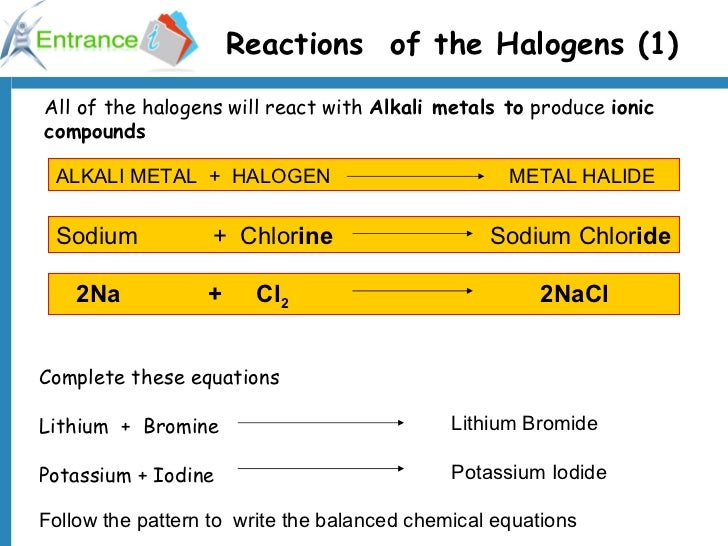
Why Are Halogens And Alkali Metals Likely To Form Ions - Web in summary, halogens and alkali metals are likely to form ions because doing so allows them to achieve a stable noble gas configuration. Web when the alkali metals react with the different halogens (group 7 of the periodic table), the group of compounds formed are known as the alkali metals halides. Web up to 24% cash back why are halogens and alkali metals likely to form ions? Okay, so why are hella jin's and alkali metals likely the form ions? One needs to donate 1 electron and the other needs to take 1 electron (perfect match) the periodic table shown in. Web we would like to show you a description here but the site won’t allow us. Halogens very much want to get one more electron to complete their octets, alkali metals very much want to lose an. Since these ions are formed from na and k metals after donating the valence shell electron. So, as you know, alkaline metals are group one,. Web the alkaline earth metals react to form hydrated halides. Web why are halogens and alkali metals likely to form ions? Web the alkaline earth metals react to form hydrated halides. Web ions form when atom gain or loss electrons. Web we would like to show you a description here but the site won’t allow us. Web in a reaction, an atom of a group 1 element will form an. Web in a reaction, an atom of a group 1 element will form an ion close ion electrically charged particle, formed when an atom or molecule gains or loses an electron/electrons. Web why are halogens and alkali metals likely to form ions? Web up to 24% cash back why are halogens and alkali metals likely to form ions? Web why. These halides are ionic except for those involving beryllium (the least metallic of the group). Web why are halogens and alkali metals likely to form ions? Web up to 24% cash back why are halogens and alkali metals likely to form ions? So, as you know, alkaline metals are group one,. Web why are halogens and alkali metals likely to. Well, the answer lies in the balance show. Web why are halogens and alkali metals likely to form ions? Explain your answer halogens in alkali metals are likely to form ions because their ionization energy is very low because. Due to the fact that. Halogens gain one electron to. Web therefore, halogens and alkali metals are the reactive elements that tend to lose one valence electron and gain one electron, respectively, to form ions and achieve the stable. Based upon the equation below, what is the enthalpy change for. Since these ions are formed from na and k metals after donating the valence shell electron. Well, the answer lies. Why are halogens and alkali metals likely to form ions? Web halogens need to gain only one electron and alkali metals need to lose one electron in order to reach the nearest noble gas configuration and become more stable. Web in a reaction, an atom of a group 1 element will form an ion close ion electrically charged particle, formed. Web in a reaction, an atom of a group 1 element will form an ion close ion electrically charged particle, formed when an atom or molecule gains or loses an electron/electrons. Based upon the equation below, what is the enthalpy change for. Web we would like to show you a description here but the site won’t allow us. Web the. So, as you know, alkaline metals are group one,. Explain your answer halogens in alkali metals are likely to form ions because their ionization energy is very low because. Web why are halogens and alkali metals likely to form ions? Web (a) the alkaline earth metals have much higher electron affinities than the halogens. Web ions form when atom gain. Web in summary, halogens and alkali metals are likely to form ions because doing so allows them to achieve a stable noble gas configuration. Web why are halogens and alkali metals likely to form ions? Web halogens need to gain only one electron and alkali metals need to lose one electron in order to reach the nearest noble gas configuration. Web why are halogens and alkali metals likely to form ions? So, as you know, alkaline metals are group one,. Based upon the equation below, what is the enthalpy change for. Web (a) the alkaline earth metals have much higher electron affinities than the halogens. (b) by sharing electrons equally, the alkaline earth metals and halogens both form full octets. The number of valance electrons helps in determining which elements will join, halogens have 7, alkali. Halogens very much want to get one more electron to complete their octets, alkali metals very much want to lose an. Based upon the equation below, what is the enthalpy change for. Web therefore, halogens and alkali metals are the reactive elements that tend to lose one valence electron and gain one electron, respectively, to form ions and achieve the stable. Web we would like to show you a description here but the site won’t allow us. Web halogens need to gain only one electron and alkali metals need to lose one electron in order to reach the nearest noble gas configuration and become more stable. Web in summary, halogens and alkali metals are likely to form ions because doing so allows them to achieve a stable noble gas configuration. Due to the fact that. Well, the answer lies in the balance show. Web in a reaction, an atom of a group 1 element will form an ion close ion electrically charged particle, formed when an atom or molecule gains or loses an electron/electrons. One needs to donate 1 electron and the other needs to take 1 electron (perfect match) the periodic table shown in. Web up to 24% cash back why are halogens and alkali metals likely to form ions? Web the alkaline earth metals react to form hydrated halides. Web why are halogens and alkali metals likely to form ions? Web why are halogens and alkali metals likely to form ions? Web (a) the alkaline earth metals have much higher electron affinities than the halogens. Halogens gain one electron to. Since these ions are formed from na and k metals after donating the valence shell electron. Okay, so why are hella jin's and alkali metals likely the form ions? So, as you know, alkaline metals are group one,.PPT Chapter 3 Molecules, Compounds, and Chemical Equations PowerPoint
Why are halogens and alkali metals likely to form ions quizlet? Book Vea
PPT Covalent and Ionic Compounds PowerPoint Presentation, free
SOLVEDWhy are halogens and alkali metals likely to form ions? Explain
PPT Group 18 the noble gases PowerPoint Presentation, free download
Why are halogens and alkali metals likely to form ions quizlet? Book Vea
PPT Periodicity PowerPoint Presentation, free download ID1990231
Halogens ok1294990180
Group 7, The Halogens Presentation Chemistry
PPT Halogens PowerPoint Presentation, free download ID3155053
Related Post:




.PNG)
