How Many Bonds Can Phosphorus Form
How Many Bonds Can Phosphorus Form - Web the four oxygen substituents in phosphate groups are arranged about the central phosphorus atom with tetrahedral geometry, however there are a total of five. Web in each case, the sum of the number of bonds and the number of lone pairs is 4, which is equivalent to eight (octet) electrons. Web this comes in handy especially when drawing lewis structures. Web phosphorus forms mostly covalent bonds. The electron configuration of the phosphorus atom can be represented by 1s22s22p63s23p3. Covalent bond two atoms form a covalent chemical bond. Phosphorus only 'needs' three more electrons to get a full valence shell of eight, but you'll notice that it actually has five valence electrons, so in. Group 6a form 2 bonds; Web how many bonds does phosphorus typically make? Group 5a form 3 bonds; Web the number of valence electrons in phosphorus is generally 5 and it requires 3 more electrons to complete its octet. Web how many bonds does phosphorus typically make? Any phosphorus rock can be used for the production of elemental phosphorus. Web phosphorus forms mostly covalent bonds. Web the four oxygen substituents in phosphate groups are arranged about the central. And group 7a form one bond. Web we would like to show you a description here but the site won’t allow us. Web there are many different modifications of phosphorus in nature. Phosphorus can fit five fluorine. The number refers to the number of bonds each. Web phosphorus forms mostly covalent bonds. The number refers to the number of bonds each. Covalent bond two atoms form a covalent chemical bond. Web apart from three and five bonds, phosphorus can also form four bonds in certain compounds. Web the number of valence electrons in phosphorus is generally 5 and it requires 3 more electrons to complete its. It’s called the honc rule, or sometimes known as honc 1234 rule. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos and images. Web phosphorus forms mostly covalent bonds. Any phosphorus rock can be used for the production of elemental phosphorus. Table showing 4 different atoms, each of their. Web information starts using one straightforward picture off the standalone covalent bond. Web in each case, the sum of the number of bonds and the number of lone pairs is 4, which is equivalent to eight (octet) electrons. Group 6a form 2 bonds; How many bonds does phosphorus typically make? Web phosphorus forms mostly covalent bonds. Web in each case, the sum of the number of bonds and the number of lone pairs is 4, which is equivalent to eight (octet) electrons. Phosphorus only 'needs' three more electrons to get a full valence shell of eight, but you'll notice that it actually has five valence electrons, so in. Group 5a form 3 bonds; Any phosphorus rock. Web we would like to show you a description here but the site won’t allow us. Table showing 4 different atoms, each of their. Group 5a form 3 bonds; Web typically, the atoms of group 4a form 4 covalent bonds; Web there are many different modifications of phosphorus in nature. Crushed phosphate rocks and sand. It’s called the honc rule, or sometimes known as honc 1234 rule. Web in each case, the sum of the number of bonds and the number of lone pairs is 4, which is equivalent to eight (octet) electrons. Web this comes in handy especially when drawing lewis structures. Group 5a form 3 bonds; Crushed phosphate rocks and sand (\(\ce{sio2}\)). Any phosphorus rock can be used for the production of elemental phosphorus. Phosphorus only 'needs' three more electrons to get a full valence shell of eight, but you'll notice that it actually has five valence electrons, so in. How many bonds does phosphorus typically make? Web there are many different modifications of phosphorus in. Web we would like to show you a description here but the site won’t allow us. Web apart from three and five bonds, phosphorus can also form four bonds in certain compounds. Table showing 4 different atoms, each of their. Web this comes in handy especially when drawing lewis structures. Web phosphorus forms mostly covalent bonds. Web typically, the atoms of group 4a form 4 covalent bonds; Table showing 4 different atoms, each of their. Group 6a form 2 bonds; Web phosphorus forms mostly covalent bonds. Any phosphorus rock can be used for the production of elemental phosphorus. Web the four oxygen substituents in phosphate groups are arranged about the central phosphorus atom with tetrahedral geometry, however there are a total of five. Web there are many different modifications of phosphorus in nature. Phosphorus only 'needs' three more electrons to get a full valence shell of eight, but you'll notice that it actually has five valence electrons, so in. Web apart from three and five bonds, phosphorus can also form four bonds in certain compounds. Phosphorus can fit five fluorine. Group 5a form 3 bonds; Web the number of valence electrons in phosphorus is generally 5 and it requires 3 more electrons to complete its octet. Any phosphorus rock can be used for the production of elemental phosphorus. And group 7a form one bond. Web we would like to show you a description here but the site won’t allow us. Web phosphorus forms mostly covalent bonds. Covalent bond two atoms form a covalent chemical bond. Web this comes in handy especially when drawing lewis structures. In these cases, it shares its lone pair of electrons with four atoms of other. Web how many bonds does phosphorus typically make?Phosphorus Definition, Facts, Symbol, Discovery, Property, Uses
LabXchange
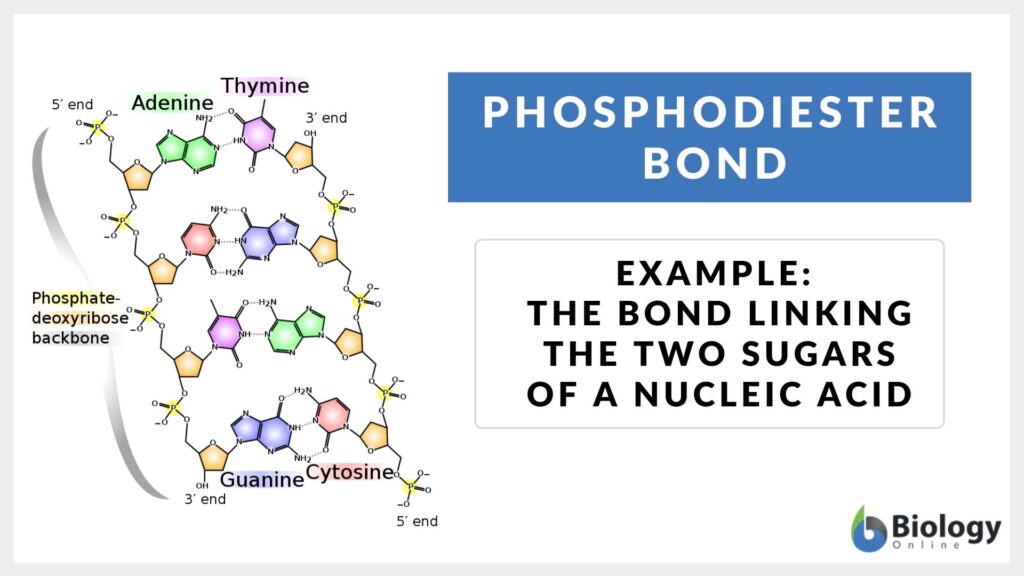
Phosphodiester bond Definition and Examples Biology Online Dictionary
How does phosphorus form 5 covalent bonds? The Unconditional Guru
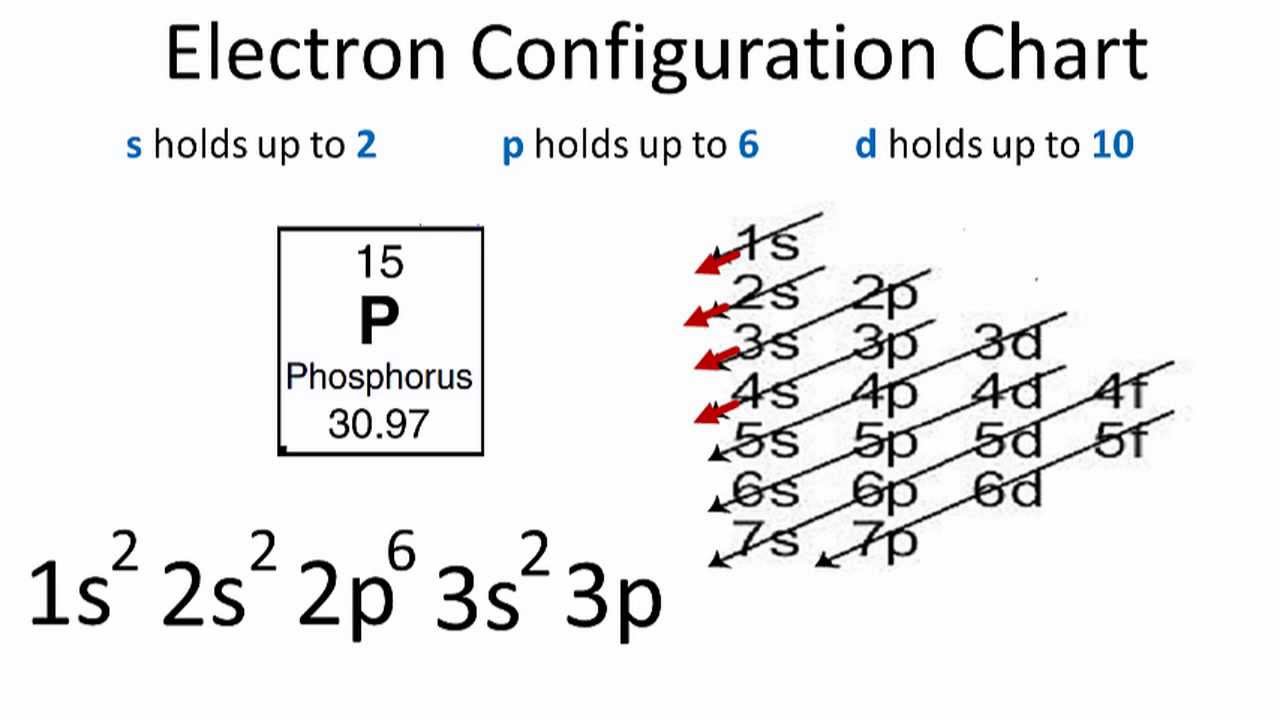
Phosphorus Electron Configuration (P) with Orbital Diagram
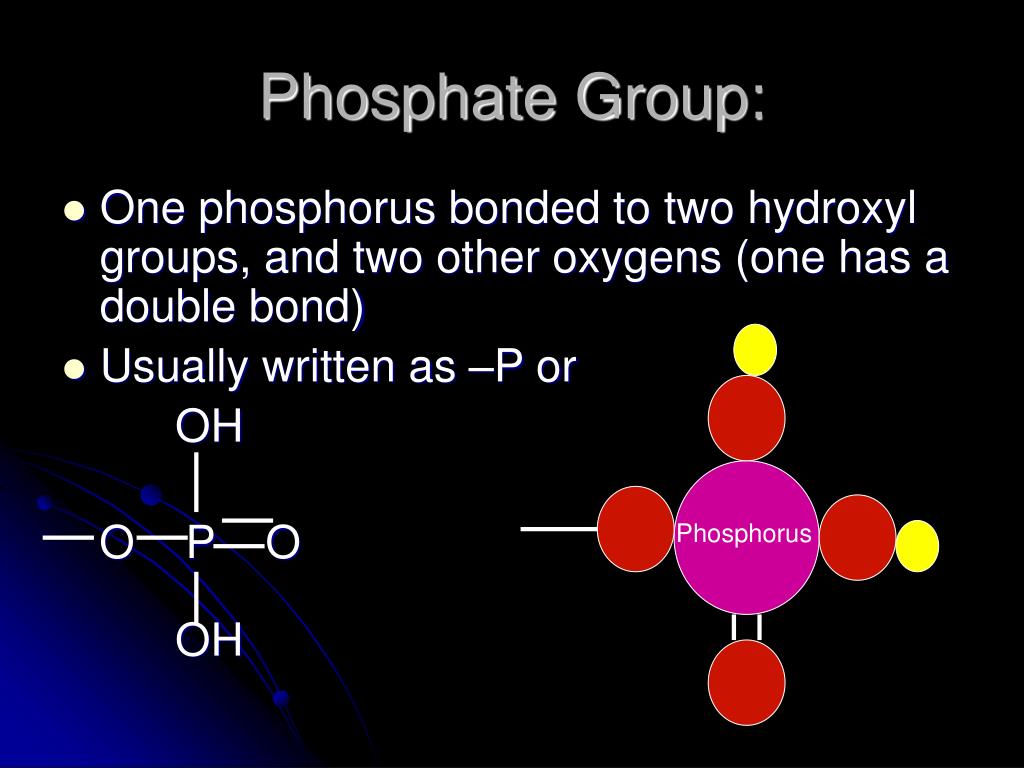
PPT Organic Molecules of Life PowerPoint Presentation, free download
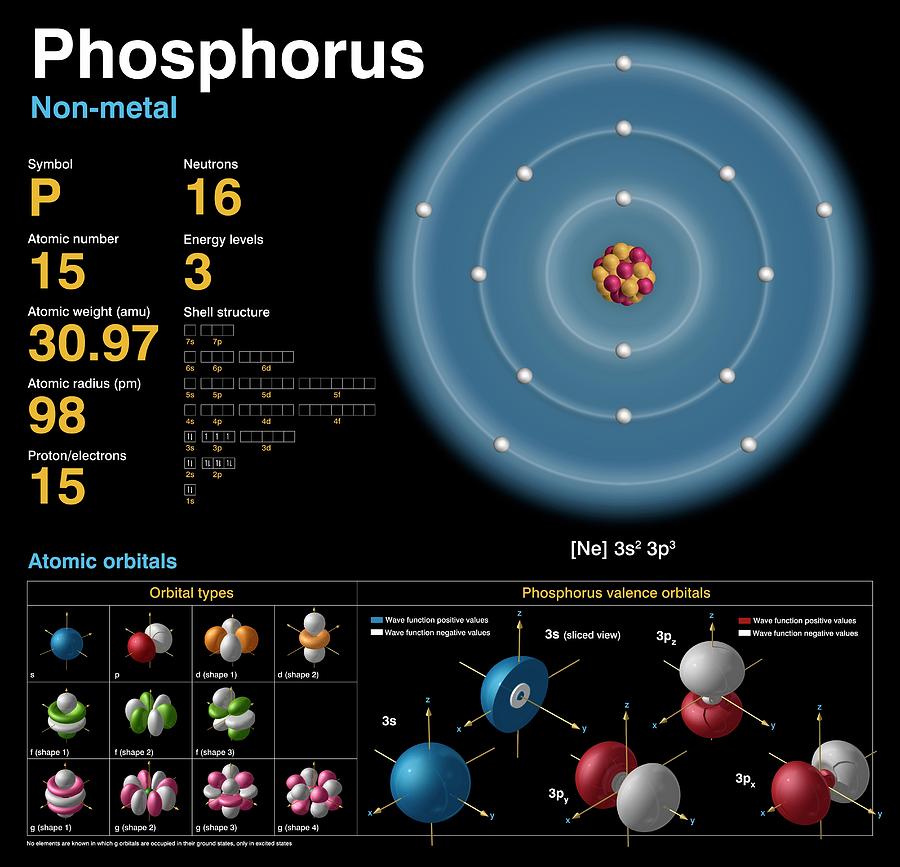
Periodic Table Phosphorus Valence Electrons Periodic Table Timeline
How Many Valence Electrons Are Found In Phosphorus
Electron Dot Diagram For Phosphorus Diagram Resource Gallery
What type of bond would form between two atoms of phosphorus? A. Triple
Related Post: