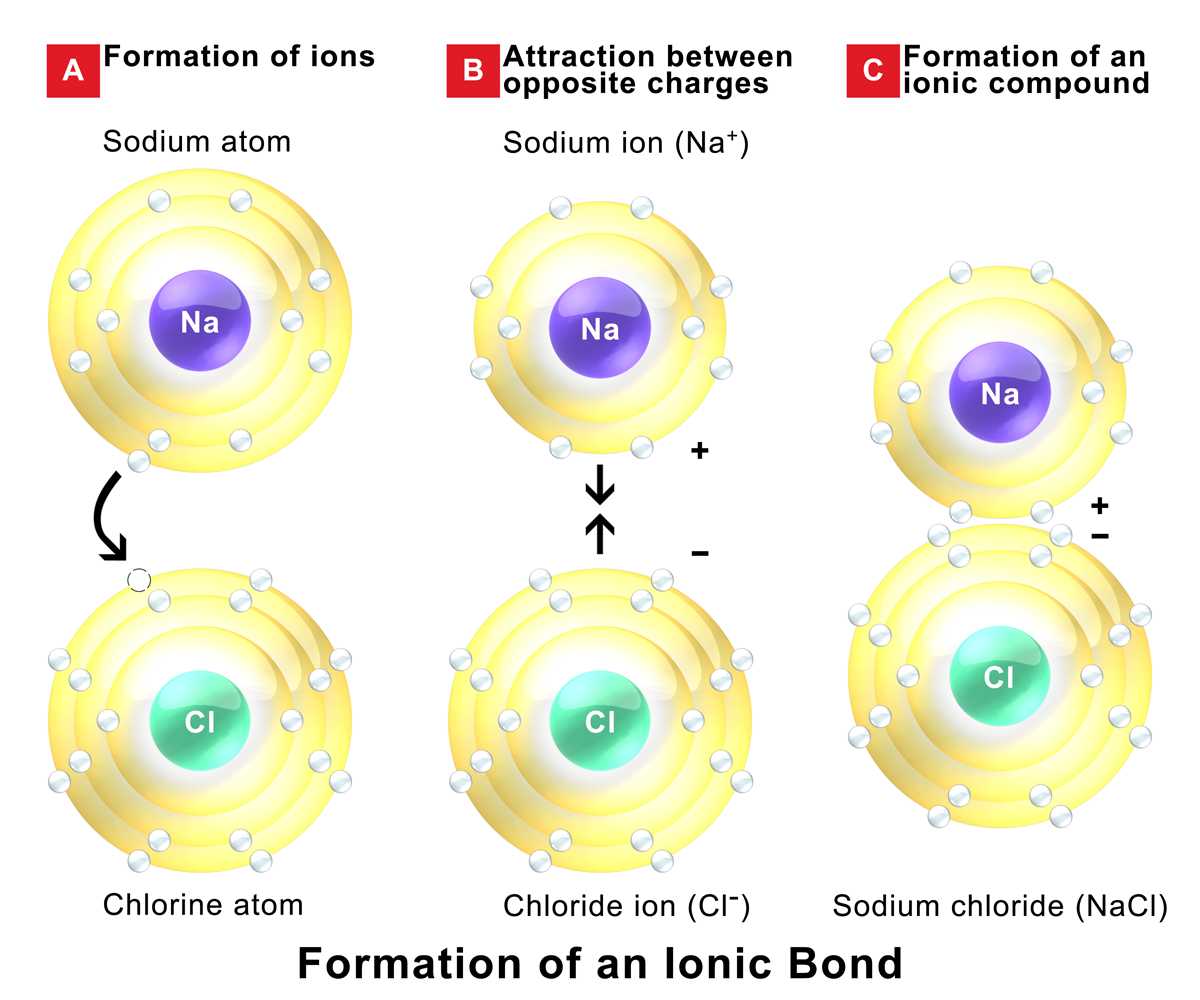
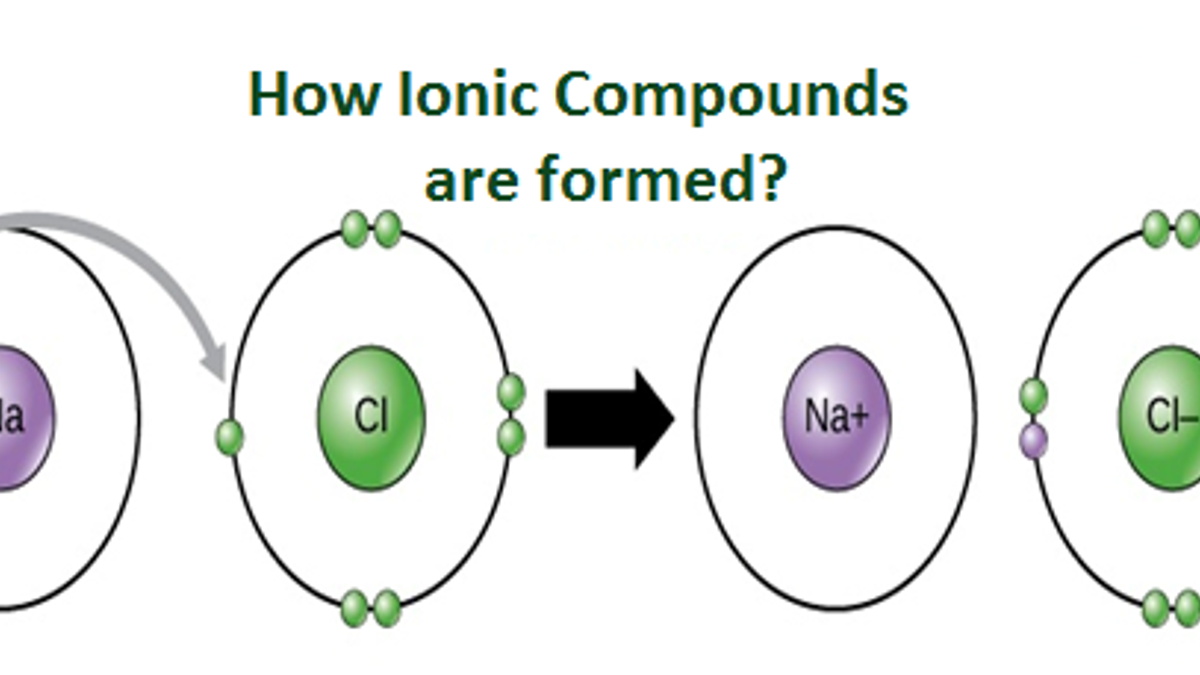
Which Pair Of Elements Will Form An Ionic Bond
Which Pair Of Elements Will Form An Ionic Bond - Web the three ions would adhere (bond) to each other by the positive/negative attraction between the ions. Ionic bonds are caused by. For example, sodium cations (positively charged ions). Among the pairs given, k and br satisfy these conditions and will form an ionic bond. Web one way to predict the type of bond that forms between two elements is to compare the electronegativities of the elements. Which property is best to use when. Write the balance equation for the. Food waste, like a feather or a bone, fall into food, causing contamination. Web 1, which pair of elements is most likely to form an ionic bond. A metal (which forms the cations) and a nonmetal (which forms the anions). Food waste, like a feather or a bone, fall into food, causing contamination. Based on their locations in the periodic table, which two elements are most likely. The transfer of electrons forms strong bonds between ions. For example, nacl is a binary ionic compound. Web the sodium atom transfers electrons to the chlorine atoms to form ionic bonds. Binary ionic compounds are composed of just two elements: Ionic bonds result from the attraction between oppositely charged ions. Web determine whether the following pairs of elements can form ionic compounds. K and br the answer is d. Food waste, like a feather or a bone, fall into food, causing contamination. Web the three ions would adhere (bond) to each other by the positive/negative attraction between the ions. A cl+cl→cl 2 b h+f→hf c na+br→nabr d o+h→h 2o medium solution verified by toppr correct option is c) sodium's ionization. Among the pairs given, k and br satisfy these conditions and will form an ionic bond. Web one way to predict the. Web determine whether the following pairs of elements can form ionic compounds. Web the pair of elements forming ionic bond is : By referring only to the. Atoms of which pair of elements will form ionic bonds in a compound? Food waste, like a feather or a bone, fall into food, causing contamination. Ionic bonds result from the attraction between oppositely charged ions. Binary ionic compounds are composed of just two elements: Which property is best to use when. (a) cl and f (b) rb and f (c) na and s (d) n and s. Web the attraction between oppositely charged ions is called an ionic bond, and it is one of the. Web one way to predict the type of bond that forms between two elements is to compare the electronegativities of the elements. In general, large differences in electronegativity. Web one type of chemical bond is an ionic bond. Web ionic bonding is the complete transfer of valence electron(s) between atoms and is a type of chemical bond that generates two. Which pair of elements will form an ionic bond? For example, nacl is a binary ionic. Which property is best to use when. Food waste, like a feather or a bone, fall into food, causing contamination. K and br the answer is d. We can think about the formation of. Binary ionic compounds are composed of just two elements: For example, nacl is a binary ionic compound. Based on their locations in the periodic table, which two elements are most likely. Food waste, like a feather or a bone, fall into food, causing contamination. Binary ionic compounds are composed of just two elements: Web ionic bonding is the complete transfer of valence electron(s) between atoms and is a type of chemical bond that generates two oppositely charged ions. Web to form ionic bonds, carbon molecules must either gain or lose 4 electrons. Web in general, covalent bonds form between nonmetals, ionic bonds form between. Web characterize bonds between the two atoms as covalent or ionic. Web binary ionic compounds are composed of just two elements: A metal (which forms the cations) and a nonmetal (which forms the anions). Web the three ions would adhere (bond) to each other by the positive/negative attraction between the ions. (a) cl and f (b) rb and f (c). Web ionic bonding is the complete transfer of valence electron(s) between atoms and is a type of chemical bond that generates two oppositely charged ions. Web binary ionic compounds are composed of just two elements: For example, nacl is a binary ionic compound. For example, nacl is a binary ionic. Ionic bonds result from the attraction between oppositely charged ions. Web characterize bonds between the two atoms as covalent or ionic. Atoms of which pair of elements will form ionic bonds in a compound? Therefore, carbon molecules share their 4 valence. For example, sodium cations (positively charged ions). Web the attraction between oppositely charged ions is called an ionic bond, and it is one of the main types of chemical bonds in chemistry. Which property is best to use when. Based on their locations in the periodic table, which two elements are most likely. Web the formation of ionic compounds. Web 1, which pair of elements is most likely to form an ionic bond. (a) cl and f (b) rb and f (c) na and s (d) n and s. Among the pairs given, k and br satisfy these conditions and will form an ionic bond. Web the three ions would adhere (bond) to each other by the positive/negative attraction between the ions. Web the sodium atom transfers electrons to the chlorine atoms to form ionic bonds. Web in general, covalent bonds form between nonmetals, ionic bonds form between metals and nonmetals, and metallic bonds form between metals. Binary ionic compounds are composed of just two elements:savvychemist Ionic Bonding (2) Dot and cross diagrams/Lewis structures
Ionic Bond Definition, Types, Properties & Examples
📐Atoms of which pair of elements will form ionic bonds in a compound
Examples of Ionic Bonds and Compounds
Ionic Bonding Presentation Chemistry
Chapter 2 Atoms, Molecules and Life Chemistry)
Ionic Bond Definition, Types, Properties & Examples
Composés ioniques Liaisons ioniques, Propriétés, Formation, Exemples
ionic bond Definition, Properties, Examples, & Facts Britannica
What are Ionic Compounds and how they are formed?
Related Post:



/ionic-bond-58fd4ea73df78ca1590682ad.jpg)
.PNG)