Which Element Is Most Likely To Form Three Covalent Bonds
Which Element Is Most Likely To Form Three Covalent Bonds - Which element is most likely to form three covalent. Web which element is most likely to form three covalent bonds? Boron commonly makes only three covalent bonds, resulting in only six. Which element is most likely to form three covalent bonds? A covalent bond is formed between two atoms by sharing electrons. One lone pair and three unpaired electrons. A) chlorineb) carbonc) oxygen d) hydrogen e) nitrogen. Incorrect question 15 which element is most likely to form three covalent bonds? Web moreover, of all the elements in the second row, carbon has the maximum number of outer shell electrons (four) capable of forming covalent bonds. Group of answer choices s n o c si. Therefore, covalent bonds lead to formation of single, double, triple, and quadruple bonds. Web science chemistry which element is most likely to form three covalent bonds? Web the most common examples are the covalent compounds of beryllium and boron. Si с p s se A covalent bond is formed between two atoms by sharing electrons. If the atoms that form a covalent bond are identical, as in h 2, cl 2, and other diatomic molecules, then the. Web nitrogen appears as n2 where there are three bonds between the two nitrogen atoms. How many covalent bonds join the atoms in a molecule of ammonia?. One lone pair and three unpaired electrons. Oxygen and other atoms. A covalent bond is formed between two atoms by sharing electrons. For example, beryllium can form two covalent bonds, resulting in only four electrons in its valence shell: The potential energy of two separate hydrogen atoms (right) decreases as. How many covalent bonds join the atoms in a molecule of ammonia?. Web which element is most likely to form three. Is determined by the distance at which the lowest potential energy is achieved. Web which element is most likely to form three covalent bonds? Web the chemical elements most likely to form covalent bonds are those that share electrons, such as carbon, as opposed to those that take them from another element to. Web the most common examples are the. Which element is most likely to form three covalent. The potential energy of two separate hydrogen atoms (right) decreases as. Is determined by the distance at which the lowest potential energy is achieved. A polar covalent bond is a. Nitrogen (n) and oxygen (o) which. Web the element which is most likely to form three covalent bonds is nitrogen. Which element is most likely to form three covalent bonds? Boron commonly makes only three covalent bonds, resulting in only six. How many covalent bonds join the atoms in a molecule of ammonia?. Web which element is most likely to form three covalent bonds? Group of answer choices s n o c si. This is because nitrogen has five electrons in its outer shell, and needs three. Web which element is most likely to form three covalent bonds? A covalent bond is formed between two atoms by sharing electrons. Web the chemical elements most likely to form covalent bonds are those that share electrons,. Web which element is most likely to form three covalent bonds? How many covalent bonds join the atoms in a molecule of ammonia?. Which group contains only elements which normally exist as diatomic molecules? Nitrogen (n) and oxygen (o) which. Incorrect question 15 which element is most likely to form three covalent bonds? Web moreover, of all the elements in the second row, carbon has the maximum number of outer shell electrons (four) capable of forming covalent bonds. Web to obtain an octet, these atoms form three covalent bonds, as in nh 3 (ammonia). Web which element is most likely to form three covalent bonds? Which element is most likely to form three. Web the most common examples are the covalent compounds of beryllium and boron. Web the element which is most likely to form three covalent bonds is nitrogen. Which element is most likely to form three covalent bonds? If the atoms that form a covalent bond are identical, as in h 2, cl 2, and other diatomic molecules, then the. A. Web the element which is most likely to form three covalent bonds is nitrogen. Web which element is most likely to form three covalent bonds? Which element is most likely to form three covalent bonds? Is determined by the distance at which the lowest potential energy is achieved. Web group 5a (15) elements such as nitrogen have five valence electrons in the atomic lewis symbol: A) chlorineb) carbonc) oxygen d) hydrogen e) nitrogen. The number of bonds an element forms in a covalent compound is determined by the. How many covalent bonds join the atoms in a molecule of ammonia?. Web science chemistry which element is most likely to form three covalent bonds? A polar covalent bond is a. Web which element is most likely to form three covalent bonds? Web a bond in which the electronegativity difference between the atoms is between 0.5 and 2.1 is called a polar covalent bond. This is because nitrogen has five electrons in its outer shell, and needs three. Group of answer choices s n o c si. Incorrect question 15 which element is most likely to form three covalent bonds? Which group in the periodic table is most likely to contain the element x in the molecule whose dot. Si с p s se Web the chemical elements most likely to form covalent bonds are those that share electrons, such as carbon, as opposed to those that take them from another element to. The potential energy of two separate hydrogen atoms (right) decreases as. One lone pair and three unpaired electrons.Reading Covalent Bonds Biology I
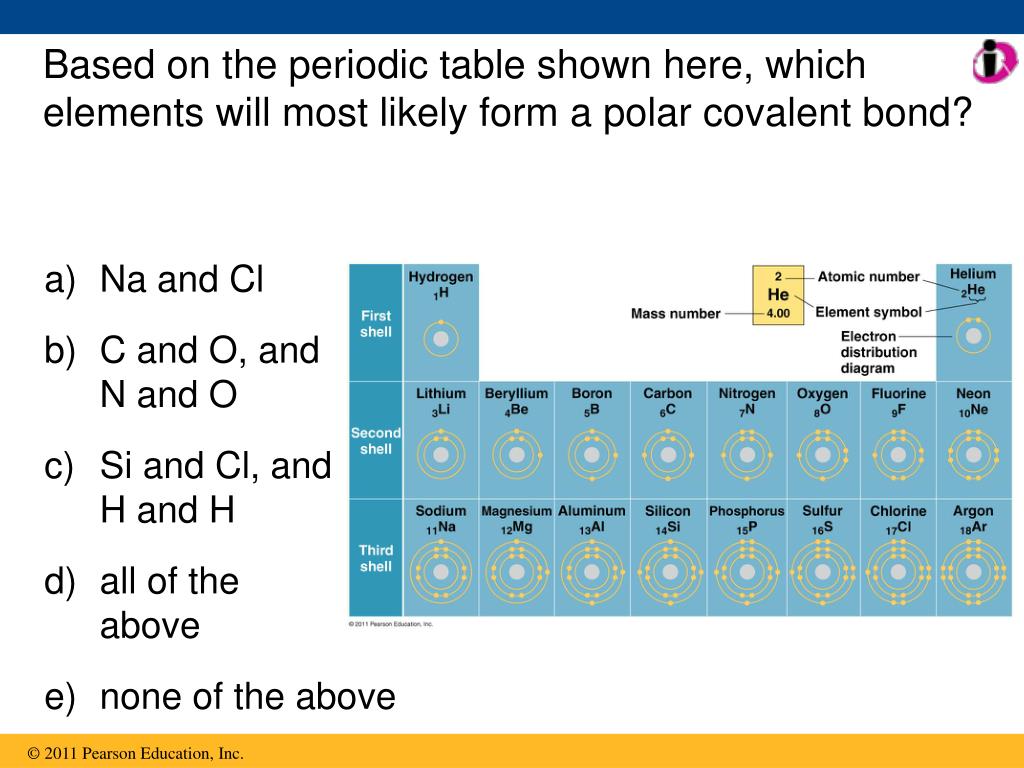
PPT Covalent Bonds PowerPoint Presentation, free download ID6647183
Which compound is most likely formed using covalent bonds? A.SiO2 B
Covalent Bond Biology Dictionary
⚗️Which diagram shows how the covalent bonds most likely form in a
Covalent Bonding (Biology) — Definition & Role Expii
Ionic Bond Definition, Types, Properties & Examples
PPT The Chemical Context of Life PowerPoint Presentation, free
How does a polar bond differ from a covalent bond
covalent bond Definition, Properties, Examples, & Facts Britannica
Related Post: