What Type Of Elements Form Ionic Bonds With Metals
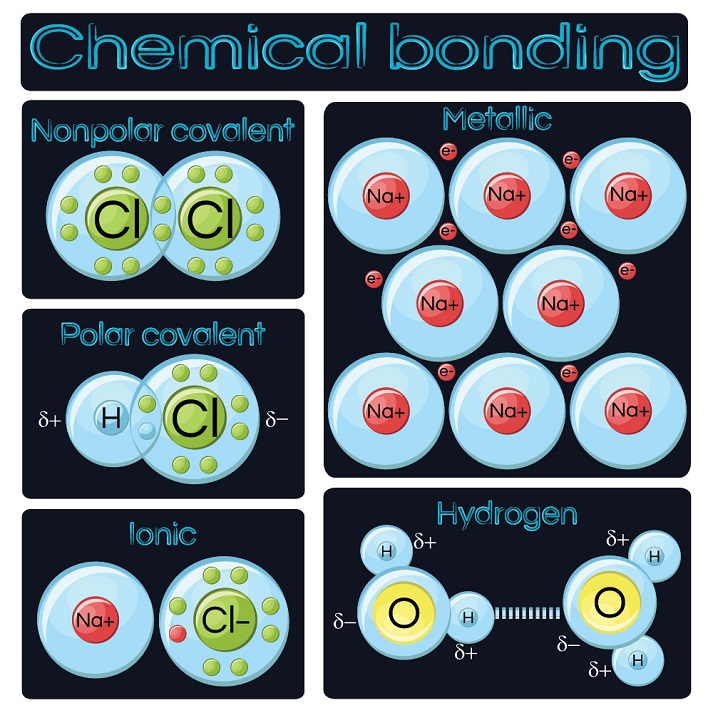
What Type Of Elements Form Ionic Bonds With Metals - Predict which forms an anion, which forms a cation, and the charges of each ion. Web ionic bonds require an electron donor, often a metal, and an electron acceptor, a nonmetal. Web , sap‑3.a (lo) , sap‑3.a.5 (ek) google classroom. Group 18 elements form none. Metals gain full outer shells by losing. One of the things you should really notice. One type of chemical bond is an ionic bond. Atoms interact with each other through the formation of chemical bonds. One way to predict the type of bond that forms between two elements is to consider whether each element is a metal or nonmetal. Web the name of a metal ion is the same as the name of the metal atom from which it forms, so ca 2+ is called a calcium ion. Web , sap‑3.a (lo) , sap‑3.a.5 (ek) google classroom. Atoms interact with each other through the formation of chemical bonds. Predict which forms an anion, which forms a cation, and the charges of each ion. Covalent bonds and ionic bonds. Metals gain full outer shells by losing. They form as a result of electrostatic attraction between oppositely charged ions and usually occur. Web ionic bonds are one of the two main types of chemical bonds. One of the things you should really notice. Web , sap‑3.a (lo) , sap‑3.a.5 (ek) google classroom. When atoms of nonmetal elements form ions, they. Ionic bonds result from the attraction. Web magnesium and nitrogen react to form an ionic compound. The metal atoms form positive ions because they. In covalent bonds, two atoms share pairs of electrons, while in ionic bonds,. Web the positive ion attracts the negative ion electrostatically thus forming an ionic bond. The metallic elements have empty. Ionic bonding is observed because metals have few electrons in. One type of chemical bond is an ionic bond. In covalent bonds, two atoms share pairs of electrons, while in ionic bonds,. Web ionic bonds form between two or more atoms by the transfer of one or more electrons between atoms. In general, covalent bonds form between nonmetals, ionic bonds form between metals and nonmetals, and metallic bonds form between metals. These oppositely charged ions attract each other to form ionic networks (or. Web the positive ion attracts the negative ion electrostatically thus forming an ionic bond. Ionic bonds result from the attraction. Group 18 elements form none. One type of chemical bond is an ionic bond. Atoms interact with each other through the formation of chemical bonds. Group 18 elements form none. Web the positive ion attracts the negative ion electrostatically thus forming an ionic bond. In covalent bonds, two atoms share pairs of electrons, while in ionic bonds,. Web the positive ion attracts the negative ion electrostatically thus forming an ionic bond. One of the things you should really notice. In covalent bonds, two atoms share pairs of electrons, while in ionic bonds,. In general, covalent bonds form between nonmetals, ionic bonds form between metals and nonmetals, and metallic bonds form between metals. Web , sap‑3.a (lo) ,. Web because they show no tendency to form negative ions, the kind of bonding present in ionic solids can immediately be ruled out. Group 18 elements form none. Web ionic bonds require an electron donor, often a metal, and an electron acceptor, a nonmetal. Web the three ions would adhere (bond) to each other by the positive/negative attraction between the. Predict which forms an anion, which forms a cation, and the charges of each ion. In general, covalent bonds form between nonmetals, ionic bonds form between metals and nonmetals, and metallic bonds form between metals. Web the name of a metal ion is the same as the name of the metal atom from which it forms, so ca 2+ is. Ionic bonding is observed because metals have few electrons in. In covalent bonds, two atoms share pairs of electrons, while in ionic bonds,. Web ionic bonds require an electron donor, often a metal, and an electron acceptor, a nonmetal. Web the three ions would adhere (bond) to each other by the positive/negative attraction between the ions. Ionic bonds result from. Predict which forms an anion, which forms a cation, and the charges of each ion. In covalent bonds, two atoms share pairs of electrons, while in ionic bonds,. These oppositely charged ions attract each other to form ionic networks (or. Group 18 elements form none. Web ionic bonds require an electron donor, often a metal, and an electron acceptor, a nonmetal. One type of chemical bond is an ionic bond. Web the positive ion attracts the negative ion electrostatically thus forming an ionic bond. Web to illustrate further, consider the two major types of chemical bonds: The metallic elements have empty. Ionic bonding is observed because metals have few electrons in. Web compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds electrostatic forces of attraction between. Web ionic bonds are one of the two main types of chemical bonds. Web because they show no tendency to form negative ions, the kind of bonding present in ionic solids can immediately be ruled out. Web ionic bonds are electrostatic bonds that are formed between metals and nonmetals. In general, covalent bonds form between nonmetals, ionic bonds form between metals and nonmetals, and metallic bonds form between metals. Web the name of a metal ion is the same as the name of the metal atom from which it forms, so ca 2+ is called a calcium ion. It is an electrostatic attraction between oppositely charged ions in a chemical. Covalent bonds and ionic bonds. One way to predict the type of bond that forms between two elements is to consider whether each element is a metal or nonmetal. The metal atoms form positive ions because they.Ionic Bond Definition, Types, Properties & Examples
Periodic Table Ions List Periodic Table Timeline
ionic bond Definition, Properties, Examples, & Facts Britannica
Naming Simple Ionic Compounds Pathways to Chemistry
Metallic Chemical Bonds Educational Resources K12 Learning, Chemistry
Ionic Bond Definition, Types, Properties & Examples
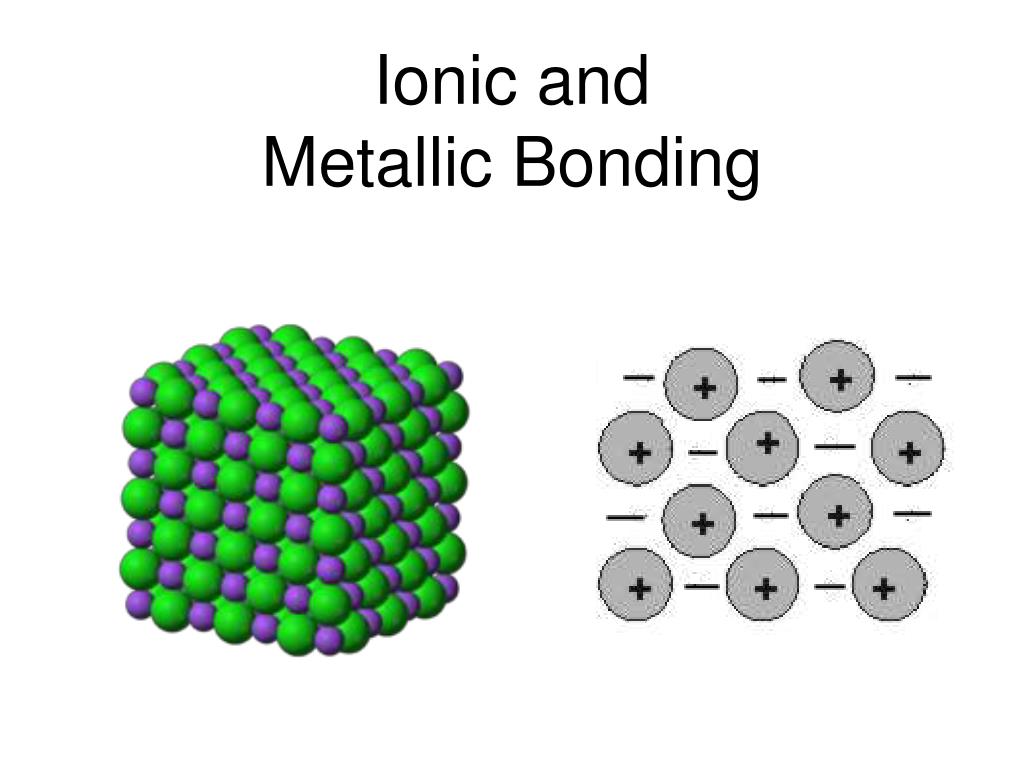
PPT Ionic and Metallic Bonding PowerPoint Presentation, free download
Ionic bonding Wikipedia
Ionic Bonding Presentation Chemistry
Ionic bond Definition, Properties, Examples, & Facts Britannica
Related Post:






.PNG)
