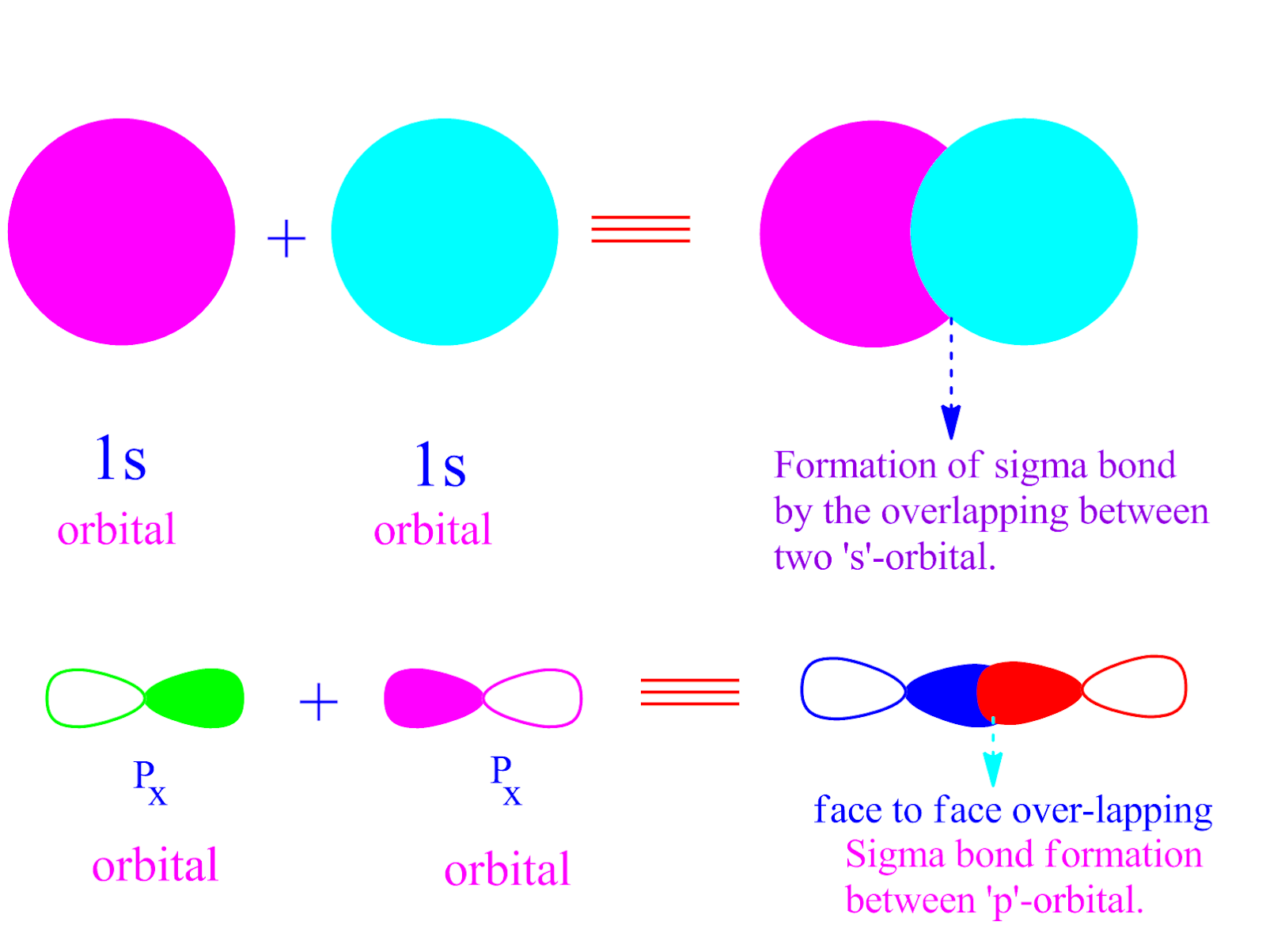
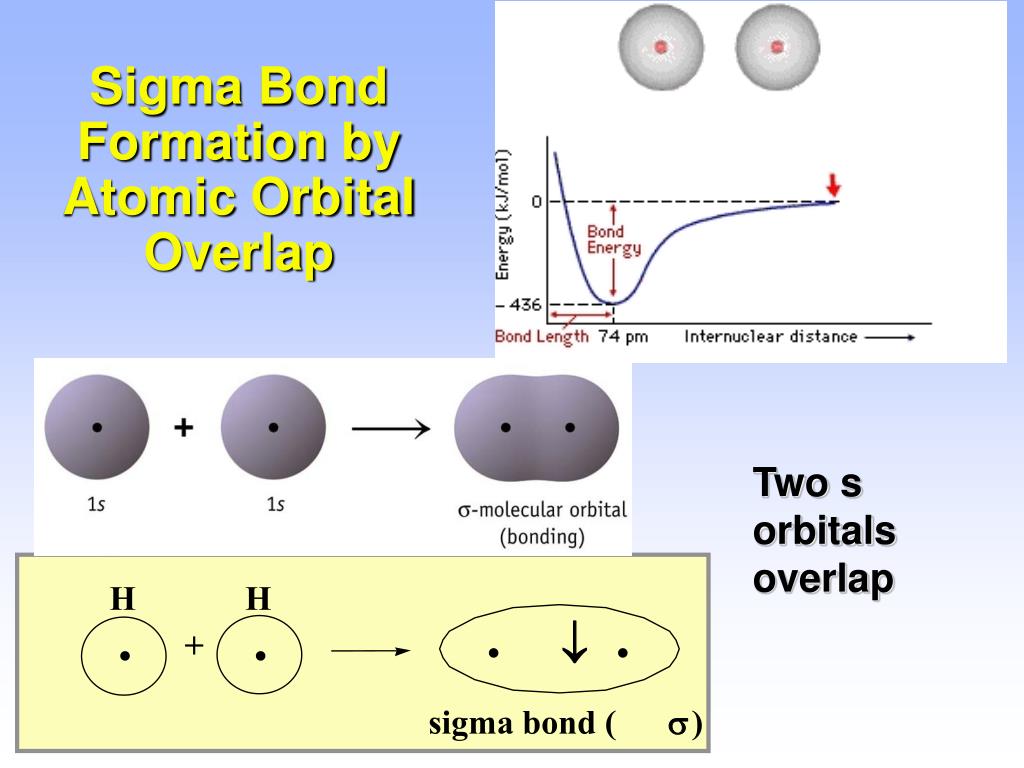
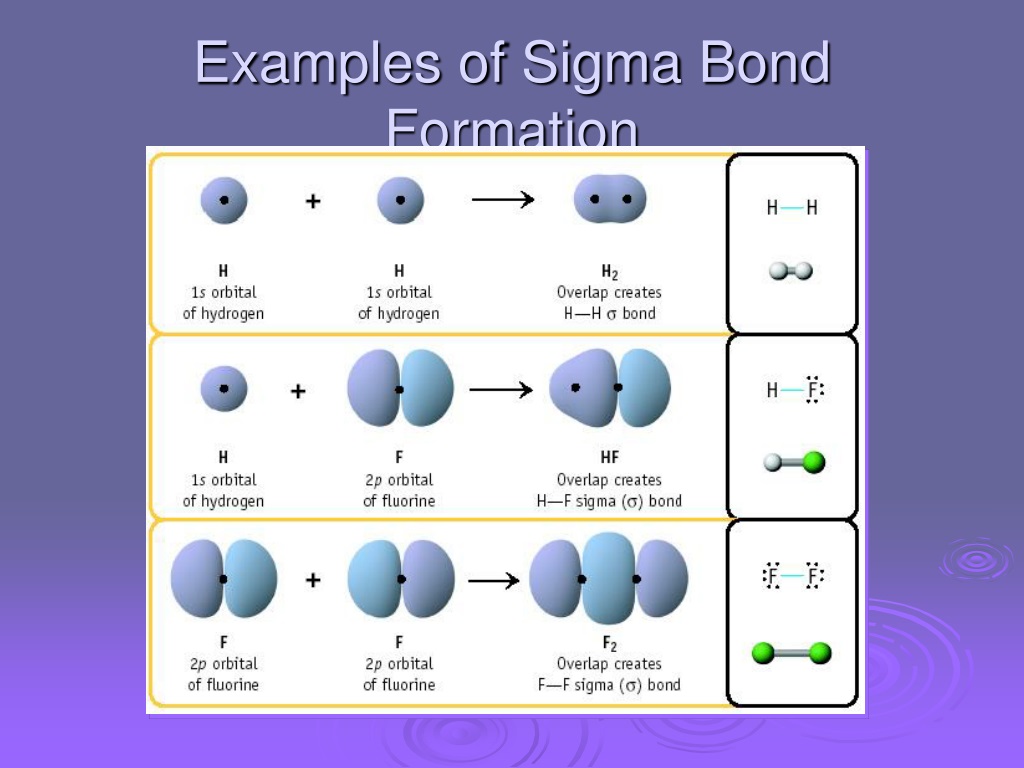
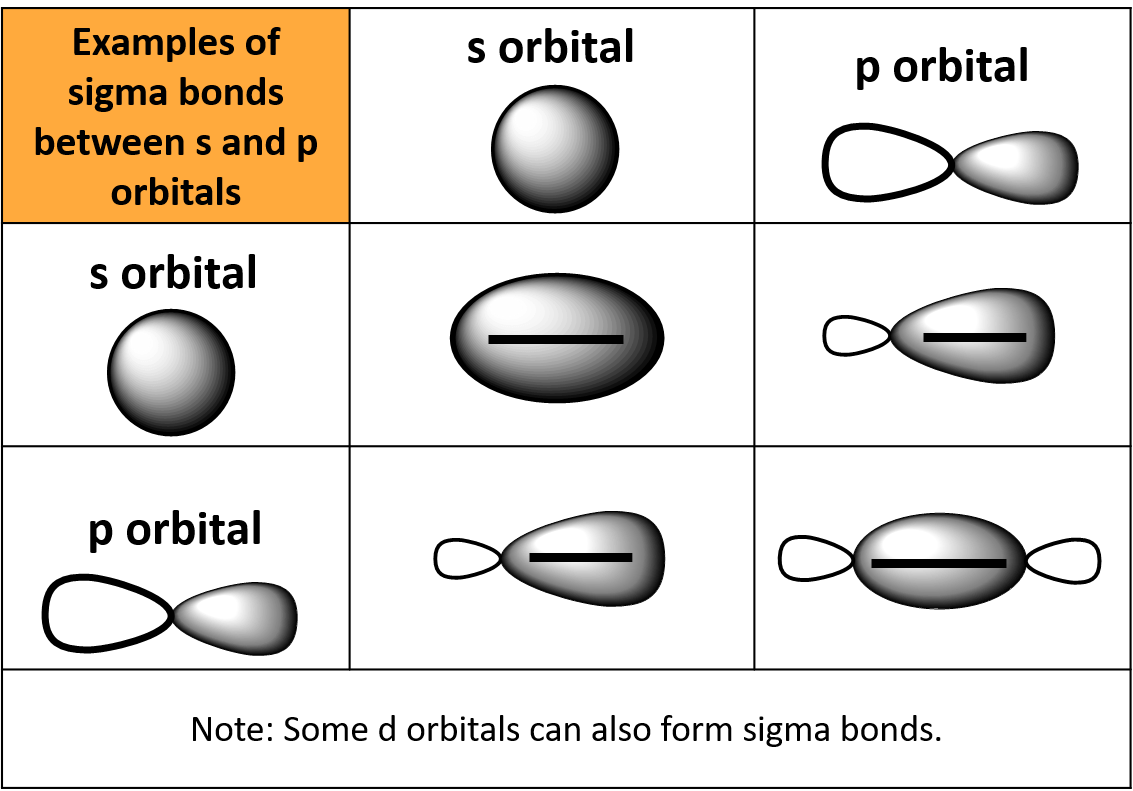
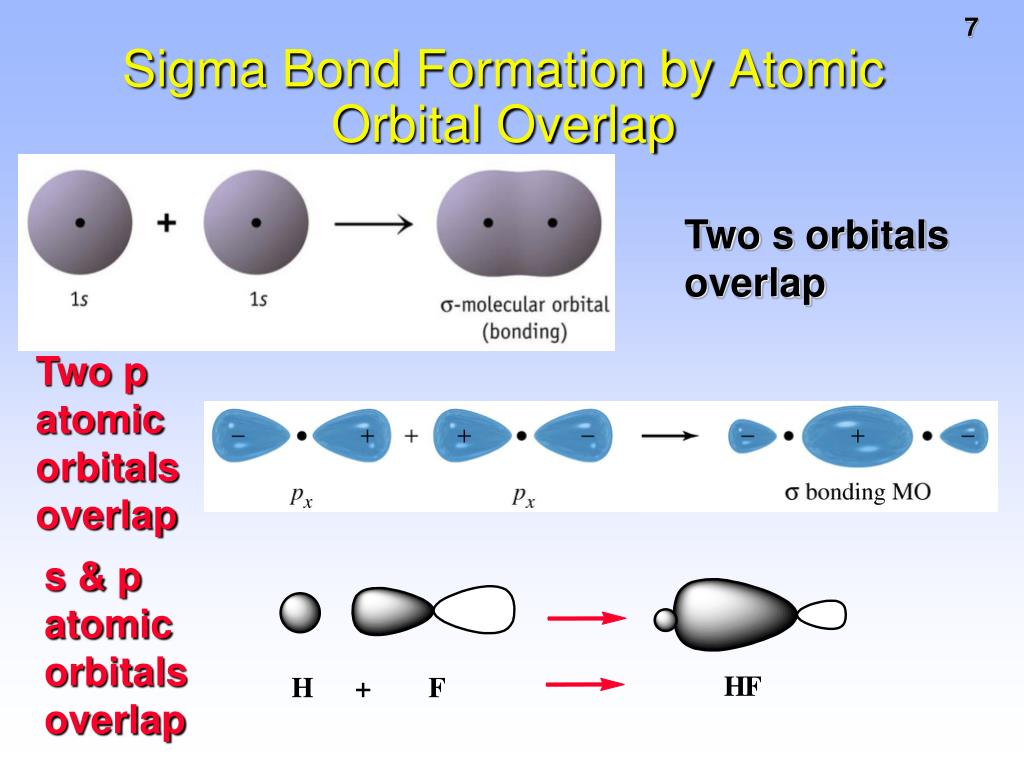
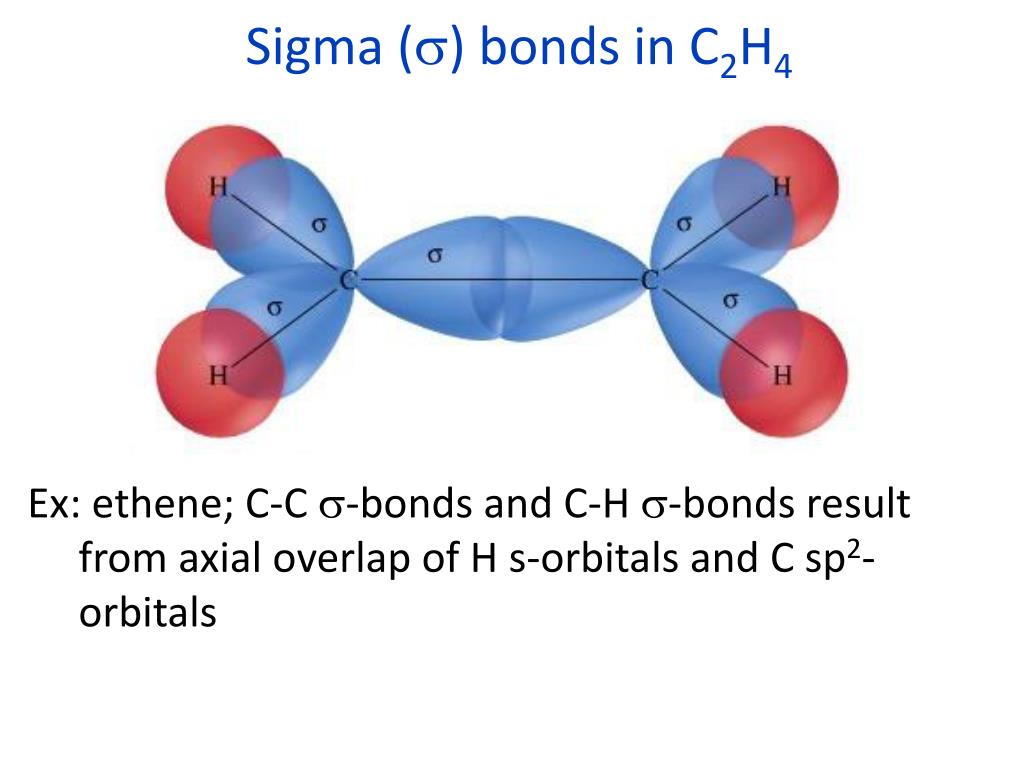
What Orbitals Form Sigma Bonds
What Orbitals Form Sigma Bonds - Examples of orbitals with appropriate symmetry are the \(s\). The unhybridized p orbitals on each carbon. Sigma bonding is most simply defined for diatomic molecules using the language and tools of symmetry groups. In chemistry, sigma bonds (σ bonds) are the strongest type of covalent chemical bond. Web illustrated glossary of organic chemistry. Web sigma bonds with sp 3 hybrid orbitals atoms that have 4 bonds, 3 bonds and 1 lone pair, 2 bonds and 2 lone pairs, or 1 bond and 3 lone pairs need four hybrid orbitals 109. Neither atomic orbitals nor molecular orbitals really exist so they. Only one sigma bond can exists between two atoms. Web the orbitals that overlap to form the sigma bonds must overlap head to head or end to end. Web the first bond between two atoms is always a sigma bond and the other bonds are always pi bonds. The bonding molecular orbital component of a σ bond. Web a sigma bond can be formed by overlap of an s atomic orbital with a p atomic orbital. The hypothetical overlap of two of the 2p orbitals on an oxygen atom (red) with the 1s orbitals of two hydrogen atoms (blue) would produce a bond angle of 90°. The three. In chemistry, sigma bonds (σ bonds) are the strongest type of covalent chemical bond. Web sigma bonds with sp 3 hybrid orbitals atoms that have 4 bonds, 3 bonds and 1 lone pair, 2 bonds and 2 lone pairs, or 1 bond and 3 lone pairs need four hybrid orbitals 109. By this definition, common forms of si… A sigma. Sigma bonding is most simply defined for diatomic molecules using the language and tools of symmetry groups. Web then, orbitals that can capably overlap with each other to form sigma bonds include the following linear combinations: Web what orbitals are used to form the bond indicated in the following molecule? The bonding molecular orbital component of a σ bond. Hydrogen. Web \(d\) orbitals can also form \(\sigma\) bonds with other types of orbitals with the appropriate symmetry. A sigma bond can also be formed by the overlap. The hypothetical overlap of two of the 2p orbitals on an oxygen atom (red) with the 1s orbitals of two hydrogen atoms (blue) would produce a bond angle of 90°. In this orbital. A sigma bond can also be formed by the overlap. Web sp³ hybridized orbitals and sigma bonds. Web what orbitals are used to form the bond indicated in the following molecule? Web the three hybrid orbitals are used to form three sigma bonds, two with the two hydrogen atoms and one with the other carbon atom. Web a sigma bond. A sigma bond can also be formed by the overlap. Hydrogen fluoride (hf) is an example: The hybrid orbitals about a central atom always are directed at the bonded atoms. Web atomic orbitals forming molecular orbitals are a way of thinking about chemical bonding. How many sigma bonds and pi bonds are. The three p orbitals on nitrogen are all mutually perpendicular or orthogonal to each other. Web the first bond between two atoms is always a sigma bond and the other bonds are always pi bonds. Web illustrated glossary of organic chemistry. Web this type of bond is referred to as a σ (sigma) bond. Ad browse & discover thousands of. Web what orbitals are used to form the bond indicated in the following molecule? Ad browse & discover thousands of science book titles, for less. In this orbital the electron density is located along the. Web a sigma bond can be formed by overlap of an s atomic orbital with a p atomic orbital. And a hybridized orbital cannot be. Web the orbitals that overlap to form the sigma bonds must overlap head to head or end to end. Web a sigma bond can be formed by overlap of an s atomic orbital with a p atomic orbital. The three p orbitals on nitrogen are all mutually perpendicular or orthogonal to each other. Web sp³ hybridized orbitals and sigma bonds.. Web \(d\) orbitals can also form \(\sigma\) bonds with other types of orbitals with the appropriate symmetry. Web a p orbital lies along a particular axis: Hydrogen fluoride (hf) is an example: The bonding molecular orbital component of a σ bond. Sp² hybridized orbitals and pi bonds. In this orbital the electron density is located along the. The bonding molecular orbital component of a σ bond. Web a p orbital lies along a particular axis: Hydrogen fluoride (hf) is an. Web the orbitals that overlap to form the sigma bonds must overlap head to head or end to end. Web sigma bonds with sp 3 hybrid orbitals atoms that have 4 bonds, 3 bonds and 1 lone pair, 2 bonds and 2 lone pairs, or 1 bond and 3 lone pairs need four hybrid orbitals 109. Web this type of bond is referred to as a σ (sigma) bond. The three p orbitals on nitrogen are all mutually perpendicular or orthogonal to each other. Sp² hybridized orbitals and pi bonds. The hybrid orbitals about a central atom always are directed at the bonded atoms. And a hybridized orbital cannot be involved in a pi bond. Web a sigma bond can be formed by overlap of an s atomic orbital with a p atomic orbital. The unhybridized p orbitals on each carbon. Web \(d\) orbitals can also form \(\sigma\) bonds with other types of orbitals with the appropriate symmetry. Web the three hybrid orbitals are used to form three sigma bonds, two with the two hydrogen atoms and one with the other carbon atom. Only one sigma bond can exists between two atoms. Sigma bonding is most simply defined for diatomic molecules using the language and tools of symmetry groups. A sigma bond can also be formed by the overlap. By this definition, common forms of si… Web then, orbitals that can capably overlap with each other to form sigma bonds include the following linear combinations:Sigma and Pi Bonds Brilliant Math & Science Wiki
Why are sigma bond more stronger than pi bond ? PG.CHEMEASY
PPT CHAPTER 10 PowerPoint Presentation, free download ID3389911
Sigma bond gilitmetro
Sigma and Pi Bonds — Definition & Overview Expii
[Solved] sketch sigma and pi bond from p orbital Course Hero
PPT Covalent Bonds PowerPoint Presentation, free download ID3048466
Sigma and Pi Bonds — Definition & Overview Expii
PPT CHAPTER 10 PowerPoint Presentation, free download ID3389911
PPT Molecular Geometry and Bonding Theories PowerPoint Presentation
Related Post: