What Ion Does Fluorine Form
What Ion Does Fluorine Form - F + h → hf (1)
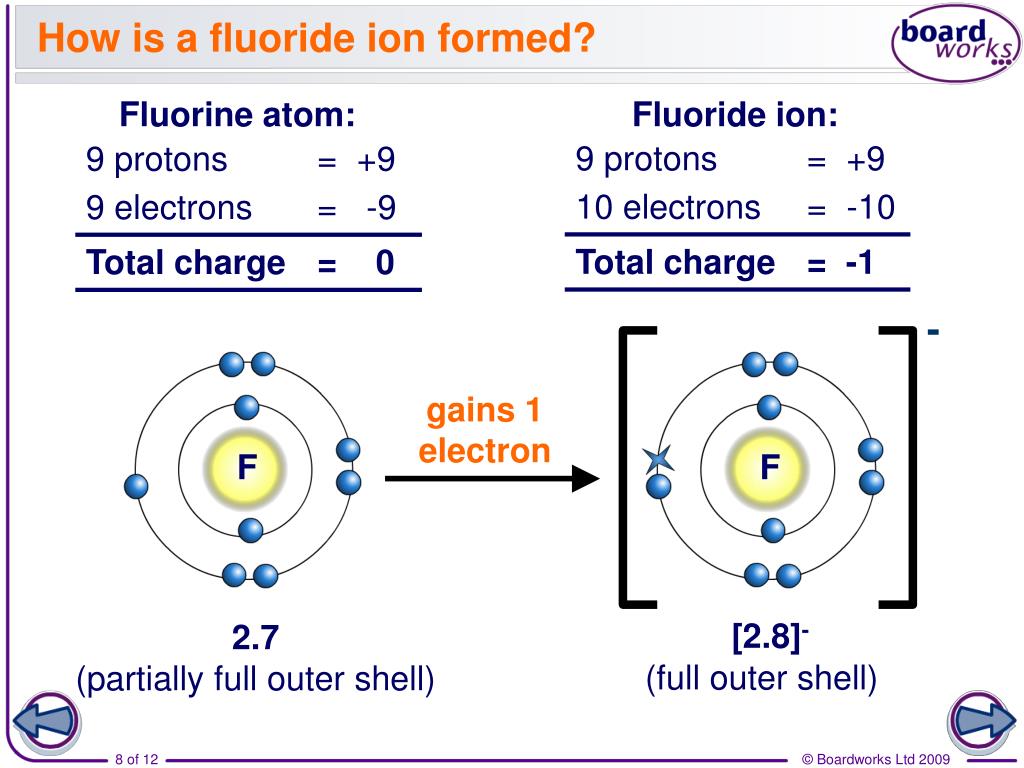
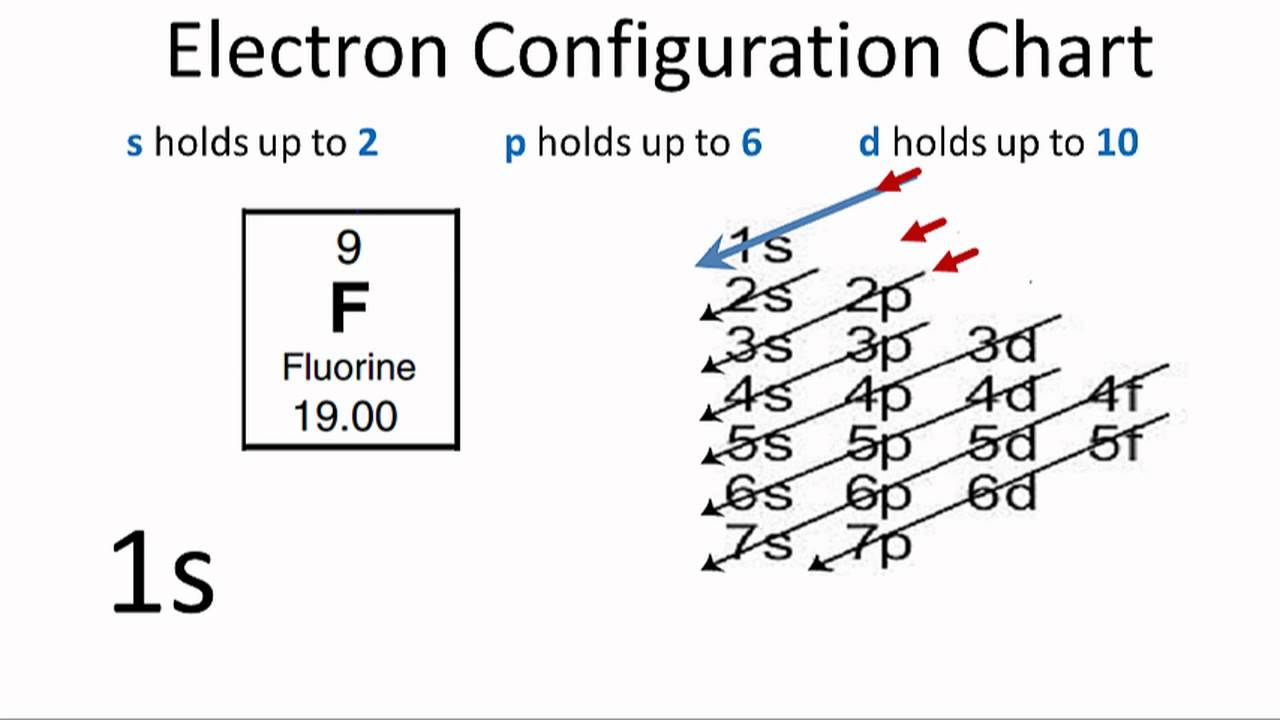
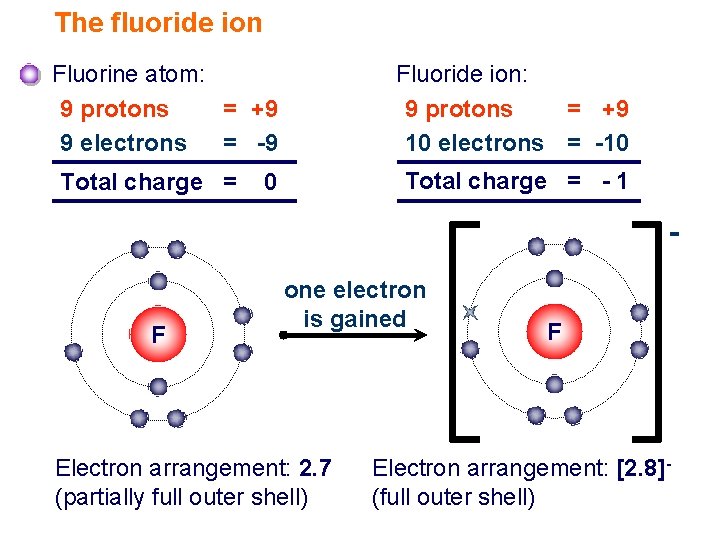
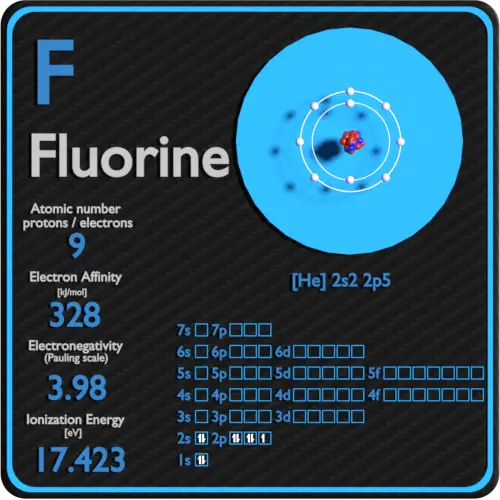
this neutralization reaction forms hydrogen fluoride (hf), the conjugate acid of fluoride. Later reports estimated 2011 global fluorochemical sales at $15 billion and pre… Since the fluoride ion is small (1.33 å) and the least polarizable anion (i.e.,. Chebi having a chemical formula of f−, fluoride. Web web with other atoms, fluorine forms either polar covalent bonds or ionic bonds. $$ 1s^22s^22p^5 $$ we see that $z=9$ and $s=2$, giving an effective nuclear charge of +7. Most frequently, covalent bonds involving fluorine atoms are single bonds, although at least. F− fluorine is a chemical element that in pure form occurs as a dimer of two fluorine atoms, f 2. This means that they are. A fluorine atom has nine protons and nine. It is therefore a weak base, and tends to remain as the fluoride ion rather than generating a substantial amount of hydrogen fluoride. Web first ionization energy of fluorine is 17.4228 ev. Web does fluorine form a +1 ion? Fluorine is a chemical element that in pure form occurs as a dimer of two fluorine atoms, f 2. Fluoride can. Most frequently, covalent bonds involving fluorine atoms are single bonds, although at least. Fluorine is a lewis acid in weak acid, which means that it. Web first ionization energy of fluorine is 17.4228 ev. Web an ionic compound is made up of charged particles, called ions. The fluorine atom has the ground state electron. Around 4.5 million tons of ore and revenue of us$550 million were generated in 2003; Web first ionization energy of fluorine is 17.4228 ev. It is therefore a weak base, and tends to remain as the fluoride ion rather than generating a substantial amount of hydrogen fluoride. This means that they are. Most frequently, covalent bonds involving fluorine atoms are. Around 4.5 million tons of ore and revenue of us$550 million were generated in 2003; Later reports estimated 2011 global fluorochemical sales at $15 billion and pre… Chebi having a chemical formula of f−, fluoride. Fluorine is the most electronegative and reactive of all elements. Fluorine is a lewis acid in weak acid, which means that it. Production has since been increasing. Web if atoms gain electrons, they become negative ions, or anions. Web taking fluorine as an example, the electron configuration is: Chebi having a chemical formula of f−, fluoride. A fluorine atom has nine protons and nine. Web an ionic compound is made up of charged particles, called ions. The presence of fluorides below 2 parts per million in drinking water is believed to prevent dental cavities. Fluorine is a lewis acid in weak acid, which means that it. The fluorine atom has the ground state electron. It is added to drinking water in some areas. Chlorofluorocarbon restrictions lowered this to 3.6 million tons in 1994; It is therefore a weak base, and tends to remain as the fluoride ion rather than generating a substantial amount of hydrogen fluoride. It is added to drinking water in some areas. It can combine with a proton ( h ): It has seven electrons in its outer shell. It can combine with a proton ( h ): Chebi having a chemical formula of f−, fluoride. $$ 1s^22s^22p^5 $$ we see that $z=9$ and $s=2$, giving an effective nuclear charge of +7. Web an ionic compound is made up of charged particles, called ions. Web if atoms gain electrons, they become negative ions, or anions. Web when a fluorine atom gains one electron, it becomes a fluoride ion with 10 negatively charged electrons and 9 positively charged protons, which gives it a 1 charge. The fluorine atom has the ground state electron. Chebi having a chemical formula of f−, fluoride. It is therefore a weak base, and tends to remain as the fluoride ion rather. Web when a fluorine atom gains one electron, it becomes a fluoride ion with 10 negatively charged electrons and 9 positively charged protons, which gives it a 1 charge. Later reports estimated 2011 global fluorochemical sales at $15 billion and pre… It is therefore a weak base, and tends to remain as the fluoride ion rather than generating a substantial. It can combine with a proton ( h ): Later reports estimated 2011 global fluorochemical sales at $15 billion and pre… Ionization energy, also called ionization potential, is the energy necessary to remove an electron from the. Around 4.5 million tons of ore and revenue of us$550 million were generated in 2003; It has a giant lattice structure with strong electrostatic forces of attraction. Fluoride can act as a base. Since the fluoride ion is small (1.33 å) and the least polarizable anion (i.e.,. It has seven electrons in its outer shell. Fluorine is a chemical element that in pure form occurs as a dimer of two fluorine atoms, f 2. The fluorine atom has the ground state electron. Web with other atoms, fluorine forms either polar covalent bonds or ionic bonds. Chlorofluorocarbon restrictions lowered this to 3.6 million tons in 1994; F + h → hf (1)
this neutralization reaction forms hydrogen fluoride (hf), the conjugate acid of fluoride. It is a pale yellow, corrosive gas, which reacts with most organic and inorganic substances. Production has since been increasing. Web first ionization energy of fluorine is 17.4228 ev. The presence of fluorides below 2 parts per million in drinking water is believed to prevent dental cavities. Web does fluorine form a +1 ion? It is a conjugate base of a hydrogen fluoride. Fluorine is the most electronegative and reactive of all elements.Diagram representation element fluorine Royalty Free Vector
PPT How do atoms form ions? PowerPoint Presentation ID7021047
Fluorine Electron Configuration With Full Orbital Diagram
Orbital Diagram For Fluorine exatin.info
PPT IONIC BONDING PowerPoint Presentation, free download ID1785078
Ionic Bonding Elements are the simplest substances There
Fluorine Periodic Table and Atomic Properties
Fluorine Diagram
PPT FLUORINE PowerPoint Presentation, free download ID2034879
The Halogens Presentation Chemistry
Related Post:





.PNG)