What Element Can Form The Most Covalent Bonds
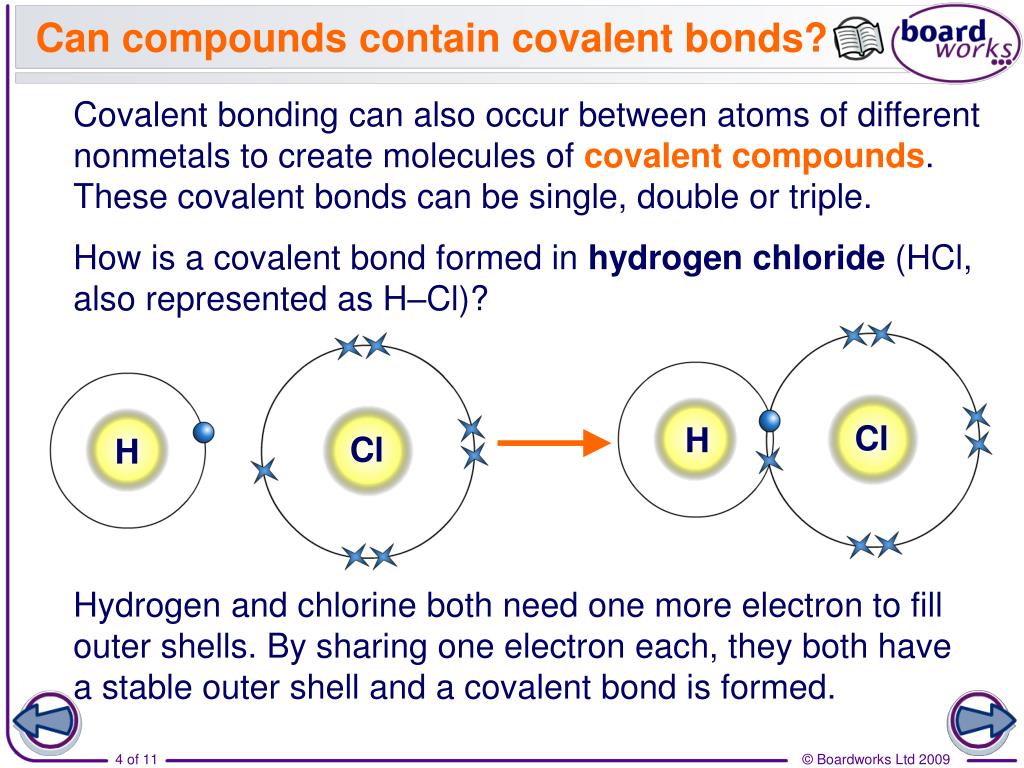
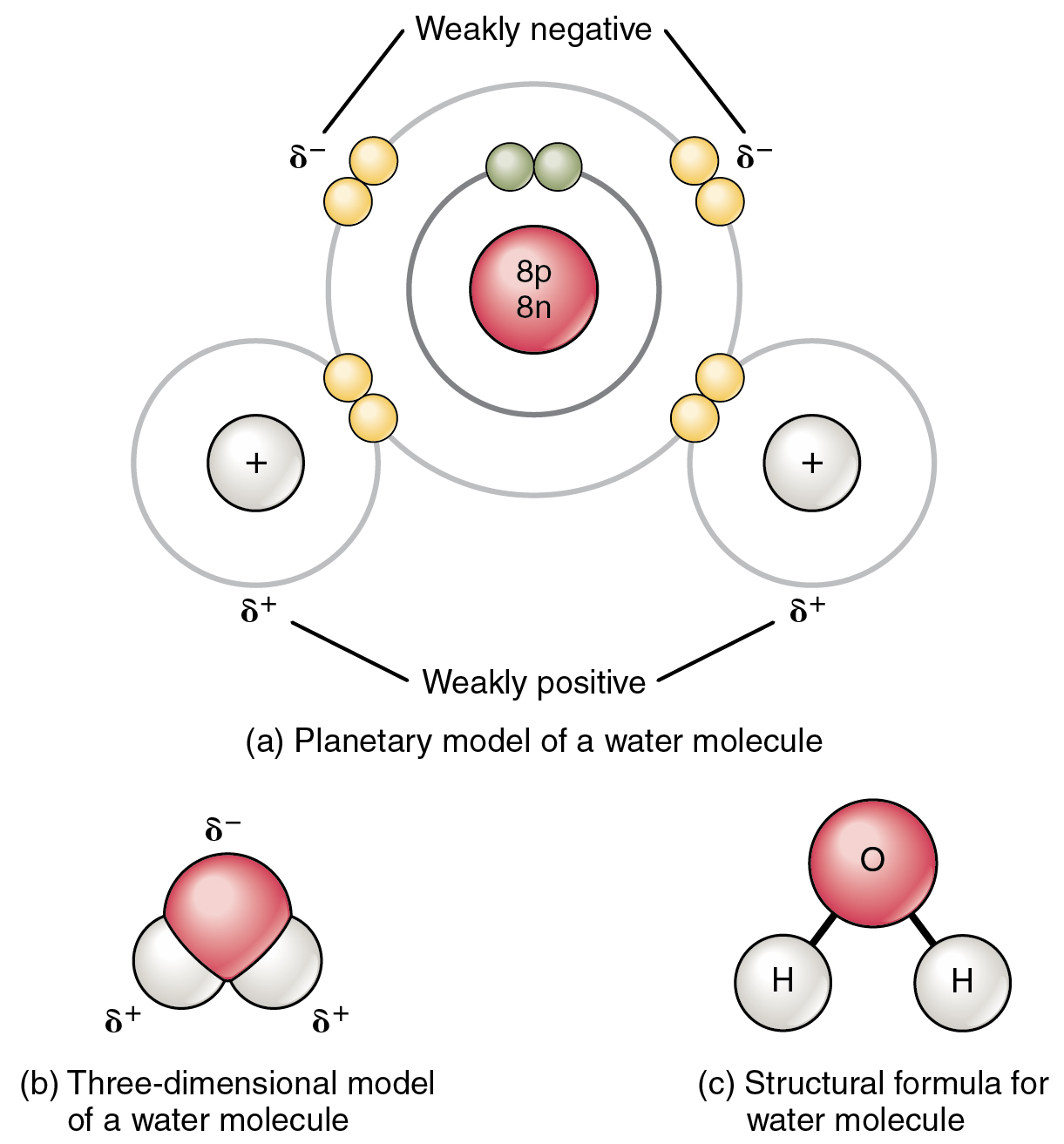
What Element Can Form The Most Covalent Bonds - The number of bonds an element forms in a covalent compound is determined by the. A covalent bond is formed by the equal sharing of electrons from both participating atoms. Web a covalent bond is formed between two atoms by sharing electrons. Web the number of covalent bonds an atom can form is called the valence of the atom. Web covalent bonds are formed between two atoms when both have similar tendencies to attract electrons to themselves (i.e., when both atoms have identical or fairly similar. This type of bonding occurs between two atoms of the same element or of elements close to each other in the. Web a covalent bond is formed between two atoms by sharing electrons. Web covalent bonding is the sharing of electrons between atoms. Nonmetal atoms frequently form covalent bonds with other nonmetal atoms. Web we would like to show you a description here but the site won’t allow us. Web moreover, of all the elements in the second row, carbon has the maximum number of outer shell electrons (four) capable of forming covalent bonds. Scientists can manipulate ionic properties and these interactions in. Web formation of covalent bonds. Web covalent bonds are formed between two atoms when both have similar tendencies to attract electrons to themselves (i.e., when both. Web these bonds form when an electron is shared between two elements and are the strongest and most common form of chemical bond in living organisms. Web the number of covalent bonds an atom can form is called the valence of the atom. Electron pairs shared between atoms of equal or very similar electronegativity constitute a nonpolar covalent bond (e.g.,.. A covalent bond is formed by the equal sharing of electrons from both participating atoms. For example, the hydrogen molecule, h 2, contains a covalent bond. This type of bonding occurs between two atoms of the same element or of elements close to each other in the. Web these bonds form when an electron is shared between two elements and. Web moreover, of all the elements in the second row, carbon has the maximum number of outer shell electrons (four) capable of forming covalent bonds. The valence of a given atom is the same in most stable neutral organic compounds. Nonmetal atoms frequently form covalent bonds with other nonmetal atoms. For example, beryllium can form two covalent bonds, resulting in. Web a covalent bond is formed between two atoms by sharing electrons. Web a covalent bond is formed when two atoms share electron pairs. Web ionic bonds are important because they allow the synthesis of specific organic compounds. Web polar covalent bonds. Nonmetal atoms frequently form covalent bonds with other nonmetal atoms. Web we would like to show you a description here but the site won’t allow us. Web a covalent bond is formed between two atoms by sharing electrons. In the diagram of methane shown here, the carbon atom has a valence of. Boron commonly makes only three covalent bonds, resulting in only six. Web ionic bonds are important because they. Boron commonly makes only three covalent bonds, resulting in only six. The number of bonds an element forms in a covalent compound is determined by the number of electrons it. Web covalent bonds involve the sharing of electron pairs between atoms. Web covalent bonds are formed between two atoms when both have similar tendencies to attract electrons to themselves (i.e.,. Web covalent bonds involve the sharing of electron pairs between atoms. For example, beryllium can form two covalent bonds, resulting in only four electrons in its valence shell: A covalent bond is formed by the equal sharing of electrons from both participating atoms. The pair of electrons participating in this type of bonding is called a. Nonmetal atoms frequently form. For example, beryllium can form two covalent bonds, resulting in only four electrons in its valence shell: In the diagram of methane shown here, the carbon atom has a valence of. Web a covalent bond is formed when two atoms share electron pairs. Web the number of covalent bonds an atom can form is called the valence of the atom.. Web a covalent bond is formed between two atoms by sharing electrons. Electron pairs shared between atoms of equal or very similar electronegativity constitute a nonpolar covalent bond (e.g.,. A covalent bond is formed by the equal sharing of electrons from both participating atoms. A bond in which the electronegativity difference between the atoms is between 0.5 and 2.1 is. Web these bonds form when an electron is shared between two elements and are the strongest and most common form of chemical bond in living organisms. Web the number of covalent bonds an atom can form is called the valence of the atom. Web a covalent bond is formed between two atoms by sharing electrons. Web covalent bonding is the sharing of electrons between atoms. Web formation of covalent bonds. For example, the hydrogen molecule, h 2, contains a covalent bond. This type of bonding occurs between two atoms of the same element or of elements close to each other in the. Scientists can manipulate ionic properties and these interactions in. Web ionic bonds are important because they allow the synthesis of specific organic compounds. Electron pairs shared between atoms of equal or very similar electronegativity constitute a nonpolar covalent bond (e.g.,. Web a covalent bond is formed between two atoms by sharing electrons. The number of bonds an element forms in a covalent compound is determined by the number of electrons it. Web a covalent bond is formed when two atoms share electron pairs. For example, beryllium can form two covalent bonds, resulting in only four electrons in its valence shell: The valence of a given atom is the same in most stable neutral organic compounds. A bond in which the electronegativity difference between the atoms is between 0.5 and 2.1 is called a polar covalent bond. Web polar covalent bonds. Boron commonly makes only three covalent bonds, resulting in only six. The pair of electrons participating in this type of bonding is called a. Web lewis proposed that an atom forms enough covalent bonds to form a full (or closed) outer electron shell.The top panel in this figure shows two hydrogen atoms sharing two
CH150 Chapter 4 Covalent Bonds and Molecular Compounds Chemistry
PPT Covalent Bonds PowerPoint Presentation, free download ID6647183
chemistry picture
covalent bond Definition, Properties, Examples, & Facts Britannica
PPT Covalent bonding in hydrogen PowerPoint Presentation, free
Attractive Forces and Bonds
Chapter 5.6 Properties of Polar Covalent Bonds Chemistry LibreTexts
Covalent Bond Biology Dictionary
Covalent Bond Examples Several Examples of Covalent (molecular) Bonds
Related Post: