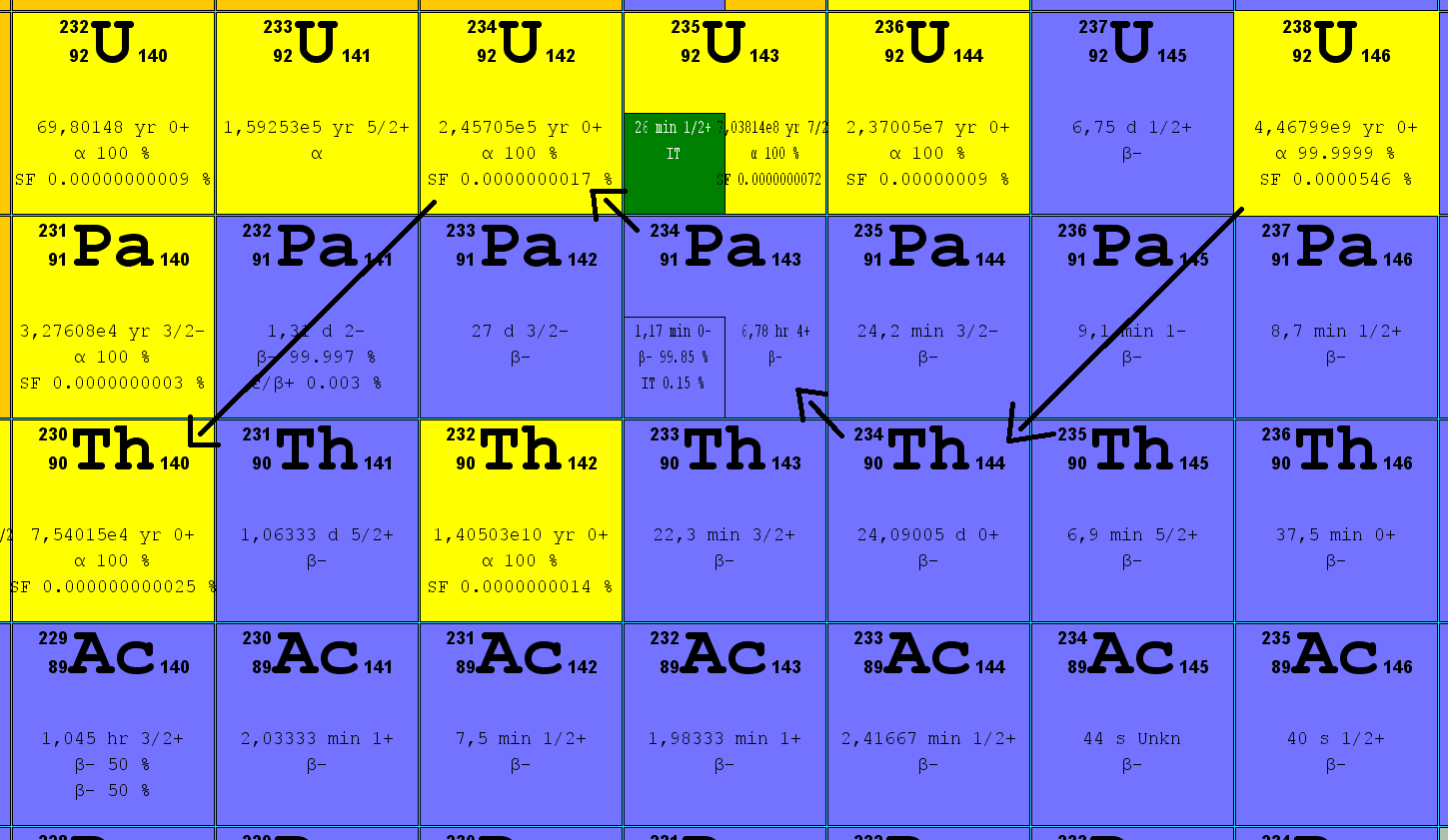
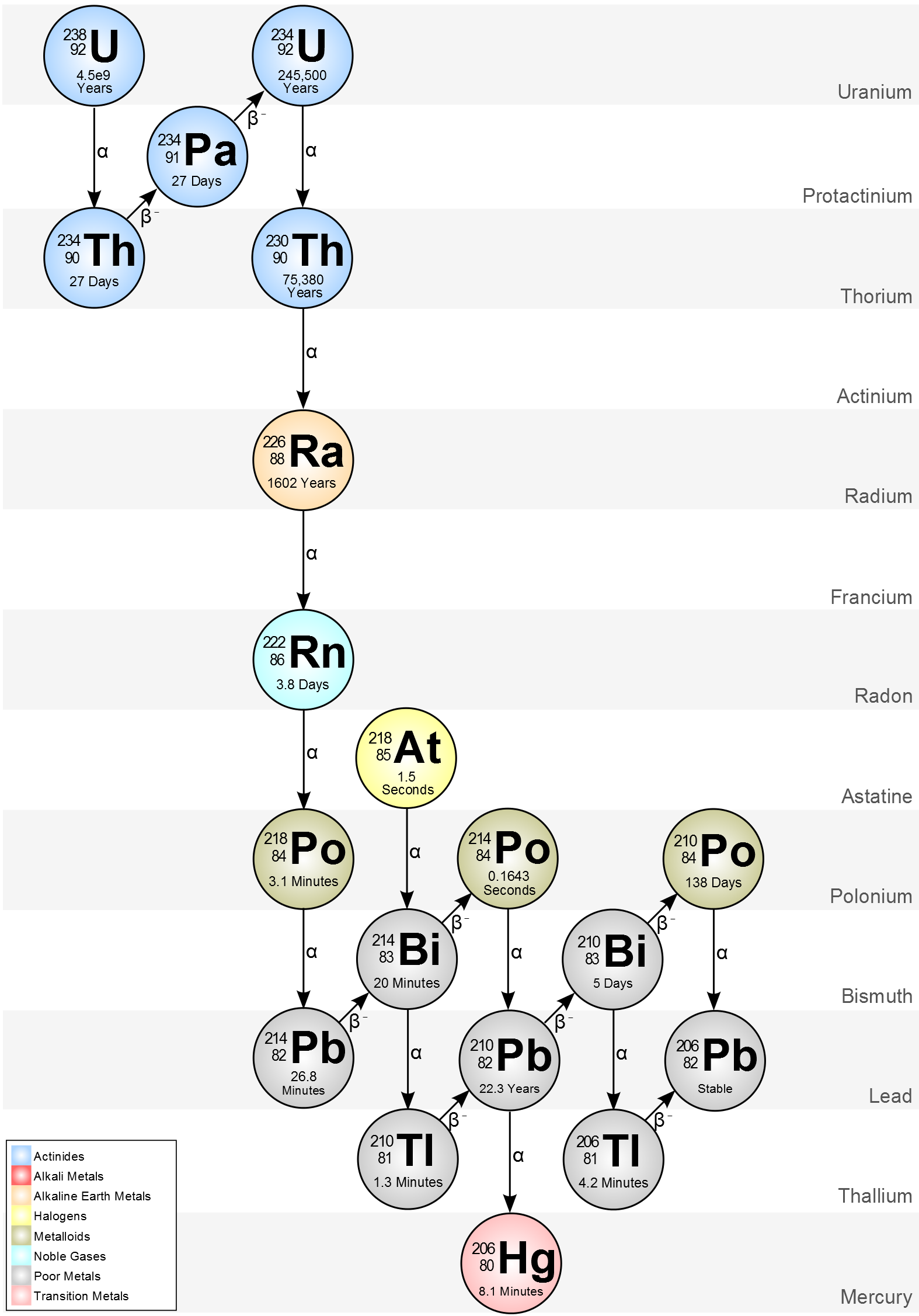
Uranium 238 Decays To Form Thorium 234
Uranium 238 Decays To Form Thorium 234 - What is the decay constant (per year)? Web we would like to show you a description here but the site won’t allow us. We know that the atomic number of uranium is equal to 92. 😎 ashishsv report flag outlined it will take 9 x 10⁹ years for 75% of the uranium. What is the decay constant (per year)? In nature, the radionuclides in these. The known masses are _2^4 he: How much is left of. How many years will it take a) 4.5 x 10° years b) 9.0. How many years will it take for 75% of the. 😎 ashishsv report flag outlined it will take 9 x 10⁹ years for 75% of the uranium. 92 238 u → 90 234 t h + 2 4 h e. How many years will it take for 75% of the. How much is left of. We know that the atomic number of uranium is equal to 92. What is the decay constant (per year)? 92 238 u → 90 234 t h + 2 4 h e. The known masses are _2^4 he: How many years will it take a) 4.5 x 10° years b) 9.0. That is, half the radioactive atoms in any sample will decay in that amount of time. 92 238 u → 90 234 t h + 2 4 h e. What is the decay constant (per year)? That is, half the radioactive atoms in any sample will decay in that amount of time. The known masses are _2^4 he: How many years will it take for 75% of the. 92 238 u → 90 234 t h + 2 4 h e. Web we would like to show you a description here but the site won’t allow us. What is the decay constant (per year)? In nature, the radionuclides in these. How many years will it take for 75% of the. In nature, the radionuclides in these. How many years will it take a) 4.5 x 10° years b) 9.0. How much is left of. What is the decay constant (per year)? The known masses are _2^4 he: The known masses are _2^4 he: How many years will it take a) 4.5 x 10° years b) 9.0. We know that the atomic number of uranium is equal to 92. What is the decay constant (per year)? In nature, the radionuclides in these. How many years will it take for 75% of the. What is the decay constant (per year)? 😎 ashishsv report flag outlined it will take 9 x 10⁹ years for 75% of the uranium. Web we would like to show you a description here but the site won’t allow us. How many years will it take a) 4.5 x 10°. What is the decay constant (per year)? The known masses are _2^4 he: That is, half the radioactive atoms in any sample will decay in that amount of time. In nature, the radionuclides in these. How many years will it take for 75% of the. Web we would like to show you a description here but the site won’t allow us. That is, half the radioactive atoms in any sample will decay in that amount of time. What is the decay constant (per year)? How much is left of. How many years will it take a) 4.5 x 10° years b) 9.0. How many years will it take a) 4.5 x 10° years b) 9.0. The known masses are _2^4 he: How much is left of. Web we would like to show you a description here but the site won’t allow us. 😎 ashishsv report flag outlined it will take 9 x 10⁹ years for 75% of the uranium. What is the decay constant (per year)? The known masses are _2^4 he: We know that the atomic number of uranium is equal to 92. 92 238 u → 90 234 t h + 2 4 h e. In nature, the radionuclides in these. 😎 ashishsv report flag outlined it will take 9 x 10⁹ years for 75% of the uranium. How many years will it take a) 4.5 x 10° years b) 9.0. Web we would like to show you a description here but the site won’t allow us. That is, half the radioactive atoms in any sample will decay in that amount of time. What is the decay constant (per year)? How much is left of. How many years will it take for 75% of the.Decay chain of the natural uranium isotopes 234 U, 235 U, and 238 U
Uranium238 Decay Chain Inspection Gallery InterNACHI®
6 Uraniumseries decay chains of the radioactive 238 U and 235 U and
uranium
Uranium 238 Properties & Uses
Uranium238 decay series. Download Scientific Diagram
Uranium235 and Uranium238 Hazardous Waste Cleanup Levels
Decay series of (A) uranium238 ( 238 U), (B) uranium235 ( 235 U), and
Uranium238 Number of Protons and Neutrons
Uranium238 decay chain from Wikipedia under CClicence. Download
Related Post: