Opdivo Enrollment Form
Opdivo Enrollment Form - Web opdivo ® (nivolumab) is indicated for the adjuvant treatment of completely resected esophageal or gastroesophageal junction cancer with residual pathologic disease in. Learn about side effects, dosage,. 3 weeks for 4 doses, then opdivo 240 mg every 2 weeks or 480 mg every 4 weeks. Healthwell foundation copay program enrollment: Ad learn more about opdivo® + chemotherapy & determine if it may help your patients. Ad find more coverage options for 2024. Ad learn more about opdivo® + chemotherapy & determine if it may help your patients. Enroll in a private medicare advantage plan today! Web form and strengths. See your medicare advantage annual open enrollment options. Web opdivo is used alone or in combination with other medicines to treat adults with: It’s given as an intravenous infusion. Web get started > my bms cases. Web these highlights do not include all the information needed to use opdivo safely and effectively. View full prescribing information, medication guide, & isi for opdivo® on physician site. Ad find more coverage options for 2024. Web form and strengths. Enroll in a private medicare advantage plan today! What is the most important information i should know about nivolumab? Web get started > my bms cases. Melanoma • adult and pediatric (12 years and older) patients with. Web these highlights do not include all the information needed to use opdivo safely and effectively. Web opdivo 1 mg/kg, followed by ipilimumab on the same day, every. Download our online enrollment user guide. What is the most important information i should know about nivolumab? You and your patient complete the applicable enrollment form. Explore the different treatment options for different types of cancer. You’ll receive opdivo at your doctor’s office or a clinic as an intravenous (iv) infusion. Ad learn about the types of treatments that may be part of your care. Ad learn more about opdivo® + chemotherapy & determine if it may. Ad learn about the types of treatments that may be part of your care. Healthwell foundation copay program enrollment: See your medicare advantage annual open enrollment options. Learn about side effects, dosage,. Web sign up for opdivo with you, a complimentary program from bms that offers helpful information about opdivo (nivolumab) and support from a care counselor. Complimentary support, tools, and educational resources for anyone considering or currently taking opdivo. 3 weeks for 4 doses, then opdivo 240 mg every 2 weeks or 480 mg every 4 weeks. Ad learn more about opdivo® + chemotherapy & determine if it may help your patients. It’s given as an intravenous infusion. Manage your bms access support ® reimbursement cases. See full prescribing information for opdivo. View full prescribing information, medication guide, & isi for opdivo® on physician site. Explore the different treatment options for different types of cancer. Web get started > my bms cases. Melanoma • adult and pediatric (12 years and older) patients with. Web get started > my bms cases. Web opdivo is used alone or in combination with other medicines to treat adults with: You’ll receive opdivo at your doctor’s office or a clinic as an intravenous (iv) infusion. Web these highlights do not include all the information needed to use opdivo safely and effectively. Opdivo is a medication used to treat. What is an explanation of benefits (eob)? Melanoma • adult and pediatric (12 years and older) patients with. Learn about side effects, dosage,. You and your patient complete the applicable enrollment form. You’ll receive opdivo at your doctor’s office or a clinic as an intravenous (iv) infusion. Complimentary support, tools, and educational resources for anyone considering or currently taking opdivo. View full prescribing information, medication guide, & isi for opdivo® on physician site. 3 weeks for 4 doses, then opdivo 240 mg every 2 weeks or 480 mg every 4 weeks. Opdivo is a medication used to treat previously treated patients with advanced renal cell carcinoma (rcc).. Ad learn about the types of treatments that may be part of your care. Ad learn more about opdivo® + chemotherapy & determine if it may help your patients. You and your patient complete the applicable enrollment form. Download our online enrollment user guide. View full prescribing information, medication guide, & isi for opdivo® on physician site. Enroll in a private medicare advantage plan today! Ad find more coverage options for 2024. Web opdivo (nivolumab) for kidney cancer. Learn about side effects, dosage,. Web form and strengths. Web access programs for opdivo® (nivolumab) patients, including bms access support and opdivo with you. Web opdivo 1 mg/kg, followed by ipilimumab on the same day, every. Eligible commercially insured patients may. Manage your bms access support ® reimbursement cases online. Web opdivo ® (nivolumab) is indicated for the adjuvant treatment of completely resected esophageal or gastroesophageal junction cancer with residual pathologic disease in. Melanoma • adult and pediatric (12 years and older) patients with. Web form more information phone: 3 weeks for 4 doses, then opdivo 240 mg every 2 weeks or 480 mg every 4 weeks. Explore the different treatment options for different types of cancer. View full prescribing information, medication guide, & isi for opdivo® on physician site.Free Aetna Prior (Rx) Authorization Form PDF eForms
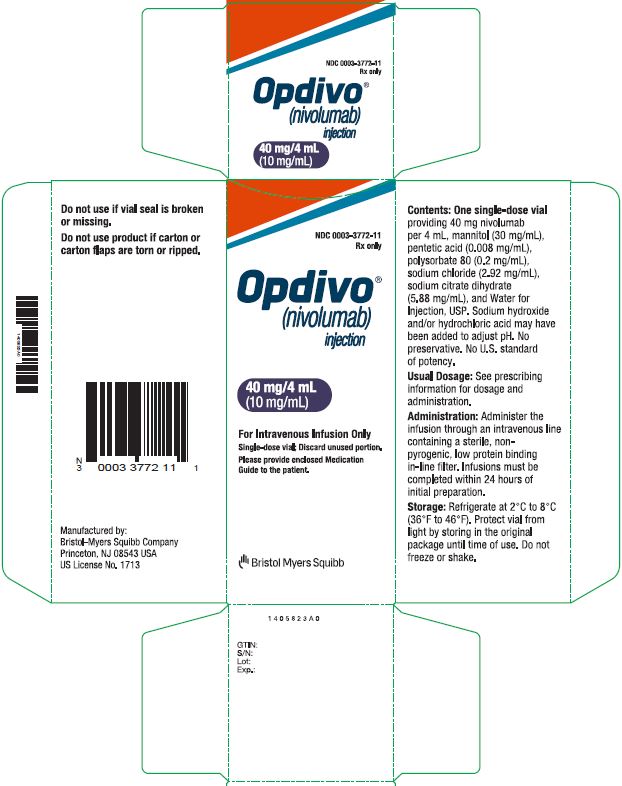
Opdivo injection Nivolumab 40mg side effect, usage
Insurance Enrollment Form Template Free Online Form Templates Create
Enrollment Form Fill Online, Printable, Fillable, Blank
Benlysta Enrollment Form Fill Online, Printable, Fillable, Blank
Humira Enrollment Form Fill Out and Sign Printable PDF Template signNow
FREE 22+ Enrollment Forms in PDF MS Word Excel
Opdivo FDA prescribing information, side effects and uses
Oklahoma Direct Deposit Enrollment Form Download Printable PDF
BristolMyers Squibb Receives Accelerated Approval of Opdivo (nivolumab
Related Post: