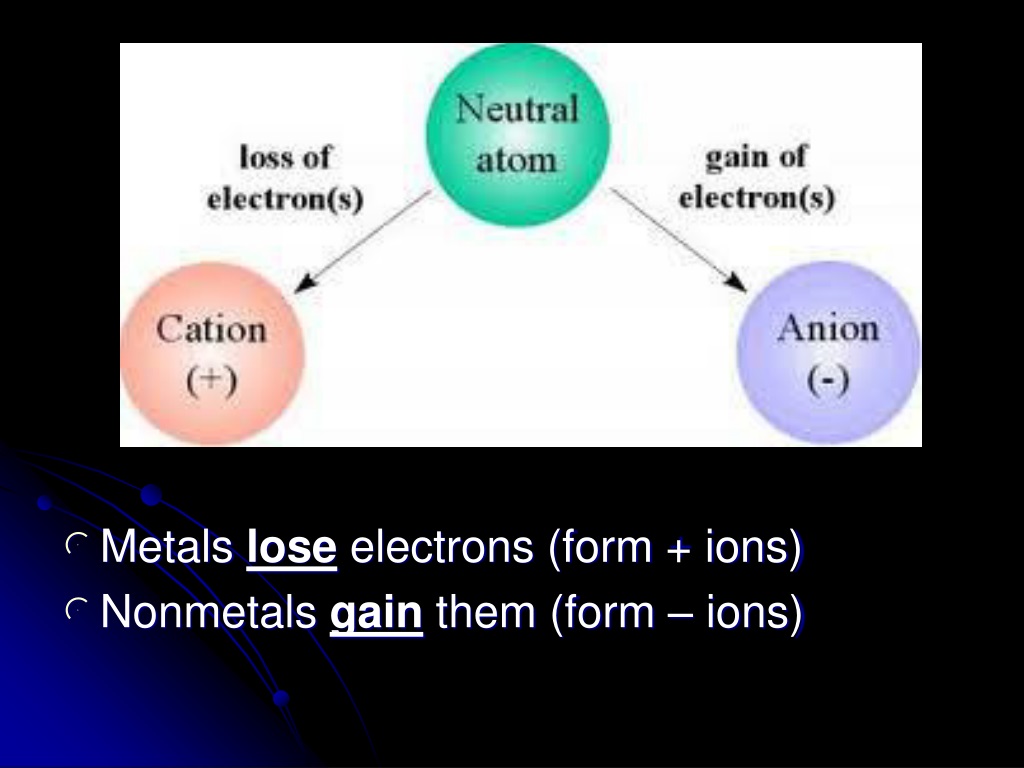
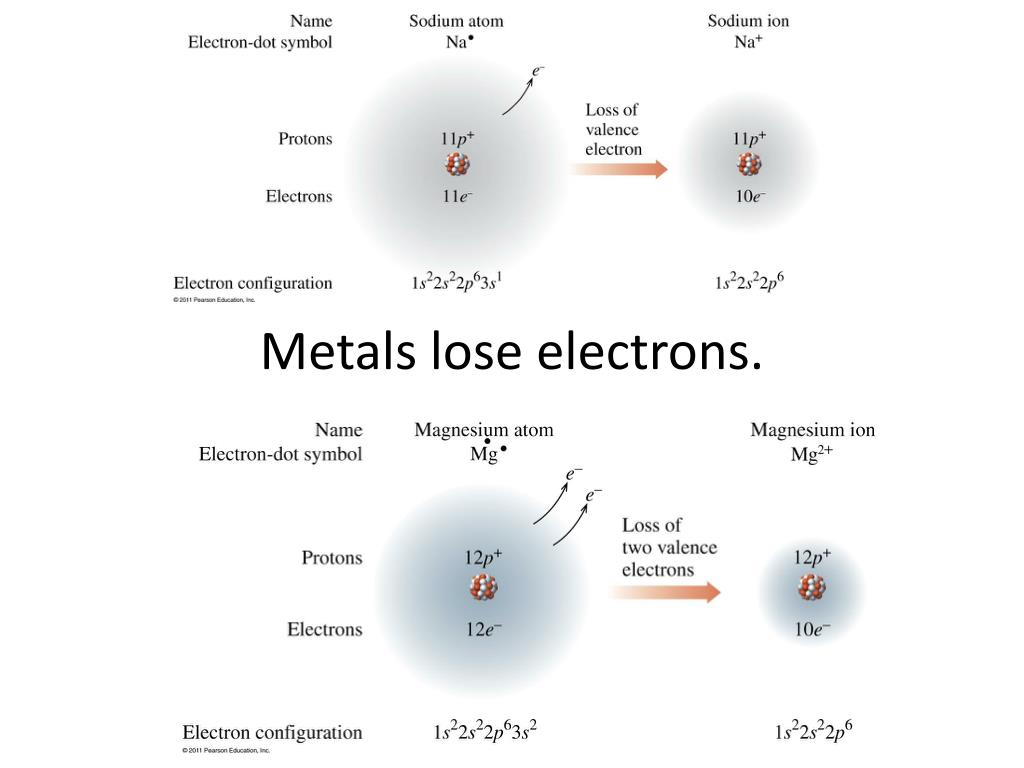
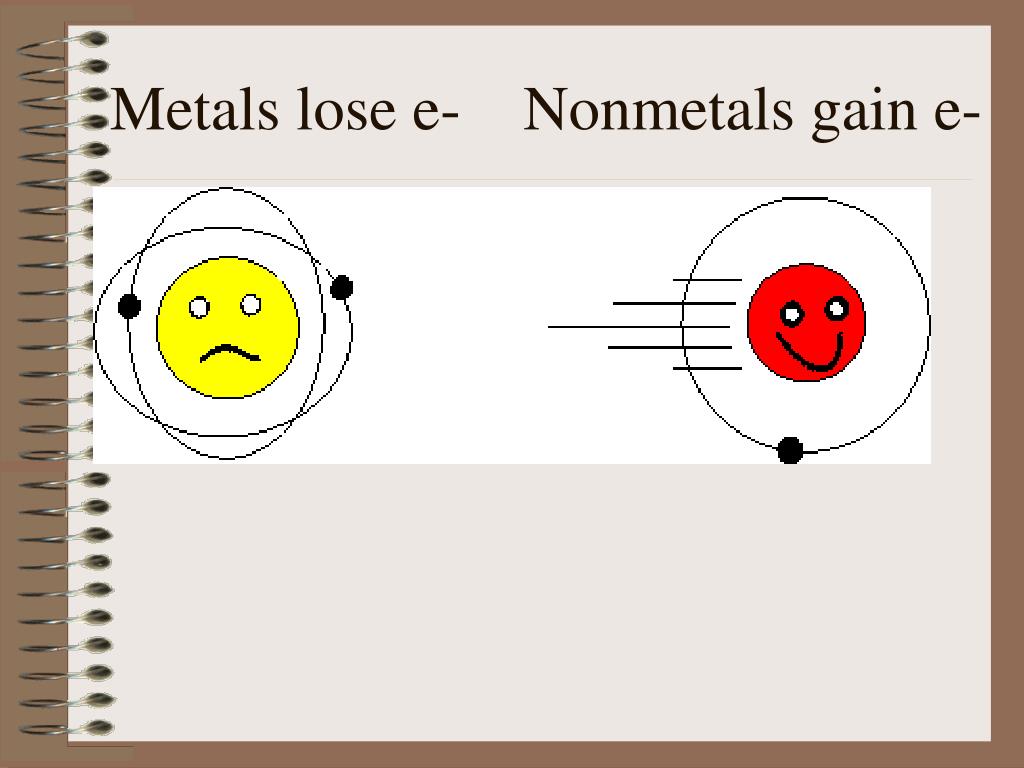
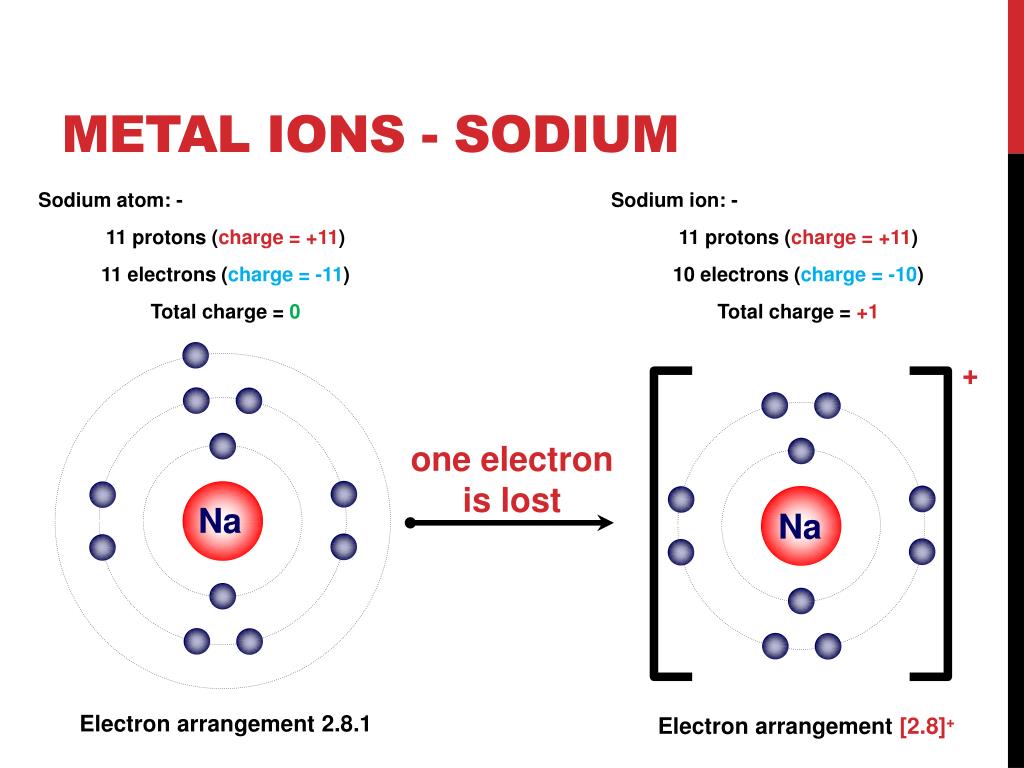
Metals Lose Electrons And Form
Metals Lose Electrons And Form - To illustrate, an atom of an. B) lose 2 electrons and form a positive ion. Metals lose their valence electrons to form cations, or positive ions. Option (a) is the correct answer. This electron loss allows metals to easily bond with other elements and create various. Metals contain more number of electrons, so in order to. Web from a modern atomic perspective, the metal stops losing ions when it reaches a reasonably stable electronic configuration. Once they lose electrons, the number of protons in their nuclei outnumber the. Metals tend to have low ionization energies, and typically lose electrons (i.e. Nomenclature of main group catlons metals tend to lose electrons and form cations in ionic compounds. Once they lose electrons, the number of protons in their nuclei outnumber the. John’s school vs osei tutu shs vs opoku ware school Metals contain more number of electrons, so in order to. Cations of the main group metals have a charge equal. B) lose 2 electrons and form a positive ion. Metals lose their valence electrons to form cations, or positive ions. Web metals tend to give away electrons to form positively charged ions while non metals tend to gain electrons to become negatively charged. Nomenclature of main group catlons metals tend to lose electrons and form cations in ionic compounds. Web atoms that lose electrons acquire a positive charge as. Metals lose their valence electrons to form cations, or positive ions. Web the researchers found that the key to enhancing ceramic toughness lay in the use of metals from the fifth and sixth columns of the periodic table, due to their. To illustrate, an atom of an. Once they lose electrons, the number of protons in their nuclei outnumber the.. The ions are positive, because they have more protons close proton subatomic particle with a positive. Web metals are electropositive elements that generally form basic or amphoteric oxides with oxygen. Metals contain more number of electrons, so in order to. Web the researchers found that the key to enhancing ceramic toughness lay in the use of metals from the fifth. Web metals (particularly those in groups 1 and 2) tend to lose the number of electrons that would leave them with the same number of electrons as in the preceding. Metals tend to have low ionization energies, and typically lose electrons (i.e. Web metals are electropositive elements that generally form basic or amphoteric oxides with oxygen. Web when a potassium. Ionic bond is formed by the transfer of electrons. Web metals lose electrons to form positive ions called cations and have a positive charge. Web metals tend to lose electrons to obtain the stable noble gas configuration of 8 valence electrons. Metals lose their valence electrons to form cations, or positive ions. Web metals tend to give away electrons to. Web atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged electrons to balance the positive charges of the. B) lose 2 electrons and form a positive ion. Web from a modern atomic perspective, the metal stops losing ions when it reaches a reasonably stable electronic configuration. Ionic bond is formed. Cations of the main group metals have a charge equal. John’s school vs osei tutu shs vs opoku ware school Nomenclature of main group catlons metals tend to lose electrons and form cations in ionic compounds. B) lose 2 electrons and form a positive ion. Metals tend to have low ionization energies, and typically lose electrons (i.e. Web atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged electrons to balance the positive charges of the. Web metals (particularly those in groups 1 and 2) tend to lose the number of electrons that would leave them with the same number of electrons as in the preceding. B) lose. Web atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged electrons to balance the positive charges of the. Once they lose electrons, the number of protons in their nuclei outnumber the. B) lose 2 electrons and form a positive ion. The ions are positive, because they have more protons close. Metals tend to have low ionization energies, and typically lose electrons (i.e. The ions are positive, because they have more protons close proton subatomic particle with a positive. Web metals tend to lose electrons to obtain the stable noble gas configuration of 8 valence electrons. Web web when metals react with other substances, the metal atoms lose electrons to form positive ions. Web atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged electrons to balance the positive charges of the. This electron loss allows metals to easily bond with other elements and create various. Web metals are electropositive elements that generally form basic or amphoteric oxides with oxygen. To illustrate, an atom of an. Web when a potassium atom reacts with a bromine atom, the bromine atom will a) lose 1 electron and form a positive ion. Web from a modern atomic perspective, the metal stops losing ions when it reaches a reasonably stable electronic configuration. Metals contain more number of electrons, so in order to. John’s school vs osei tutu shs vs opoku ware school Option (a) is the correct answer. Web metal atoms lose electrons from their outer shell when they form ions: Metals lose their valence electrons to form cations, or positive ions. Web metals are electropositive elements that generally form basic or amphoteric oxides with oxygen. Metals tend to have low ionization energies,. Nomenclature of main group catlons metals tend to lose electrons and form cations in ionic compounds. Why do they want to obtain this configuration, and how does the. B) lose 2 electrons and form a positive ion.Formation of Ion SPM Chemistry
PPT Bonding Between Atoms PowerPoint Presentation, free download ID
PPT Chemistry 120 PowerPoint Presentation, free download ID2089971
PPT Chemical Bonding PowerPoint Presentation, free download ID5674609
PPT Atoms in Combination The Chemical Bond PowerPoint Presentation
Ions of Transition Elements Mooramo
PPT THE NATURE OF MATERIALS PowerPoint Presentation, free download
Ions Predict Charge Stone Cold Chemistry Talk Ions Predict Charge
Elements P, Q, R & S have atomic numbers 11, 15, 17 & 18 respectively
PPT Ions PowerPoint Presentation, free download ID6907887
Related Post: