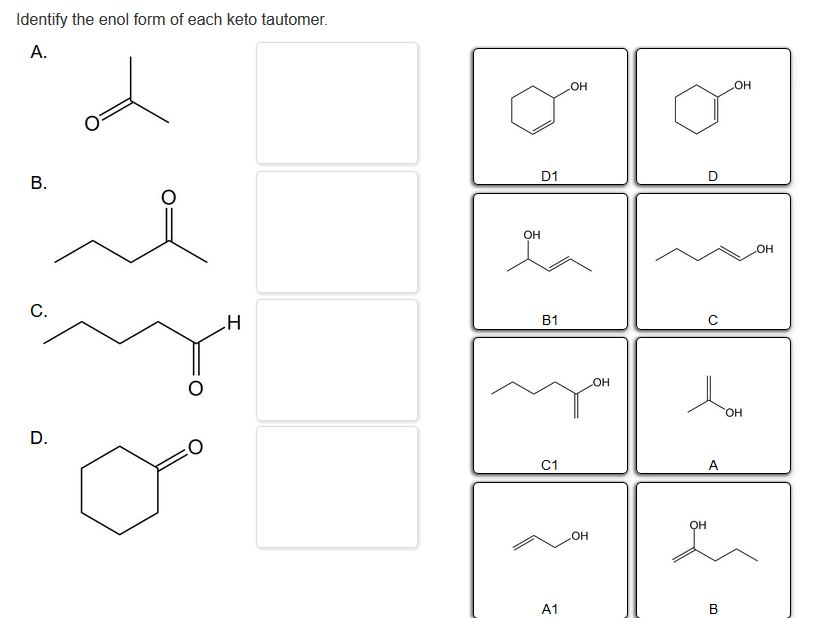
Identify The Enol Form Of Each Keto Tautomer
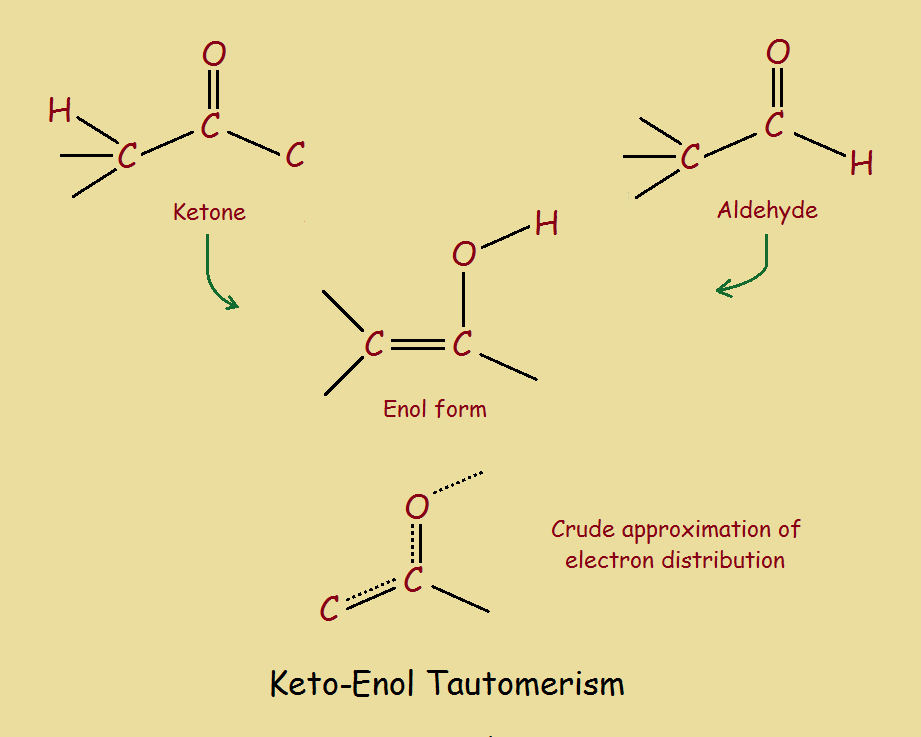
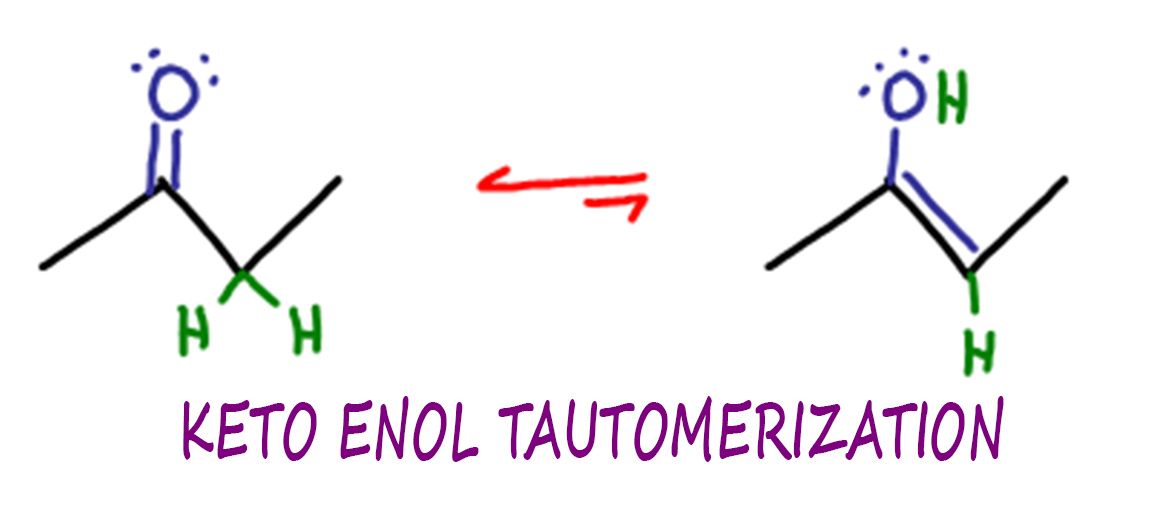
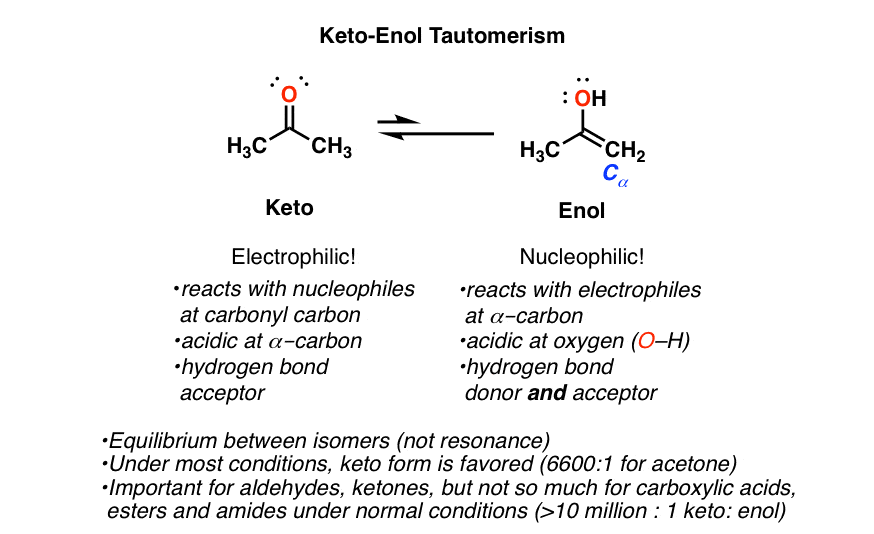
Identify The Enol Form Of Each Keto Tautomer - It can spontaneously through equilibrium get to the actual enol form. Web identify the keto form of the enol tautomer shown below: Web the keto form and the enol form, and these are different molecules. And so you could imagine,. Web which of the following have an enol form?i. If, however, protonation takes place on the. You'll get a detailed solution from a subject matter expert. The preferred enol tautomer form can be. 1) protonation of the carbonyl. A b c d this problem has been solved! Web the keto form and the enol form, and these are different molecules. They're isomers of each other so we call them tautomers and they're in equilibrium with each other. You'll get a detailed solution from a subject matter expert. Identify the enol form of each keto tautomer. It can spontaneously through equilibrium get to the actual enol form. Web if protonation of the enolate ion takes place on the α carbon, the keto tautomer is regenerated and no net change occurs. The relative stability of these two. They're isomers of each other so we call them tautomers and they're in equilibrium with each other. If, however, protonation takes place on the. Tautomers are readily interconverted constitutional isomers, usually. If, however, protonation takes place on the. They're isomers of each other so we call them tautomers and they're in equilibrium with each other. Acids and bases both bring about the. Tautomers are readily interconverted constitutional isomers, usually distinguished by a different location for an atom or a group. Identify the enol form of each keto tautomer. Web if protonation of the enolate ion takes place on the α carbon, the keto tautomer is regenerated and no net change occurs. The relative stability of these two. The preferred enol tautomer form can be. Web the keto form and the enol form, and these are different molecules. Tautomers are readily interconverted constitutional isomers, usually distinguished by a different. They're isomers of each other so we call them tautomers and they're in equilibrium with each other. Identify the enol form of each keto tautomer. Web identify the keto form of the enol tautomer shown below: The relative stability of these two. 1) protonation of the carbonyl. You'll get a detailed solution from a subject matter expert. This problem has been solved! The relative stability of these two. Web identify the keto form of the enol tautomer shown below: They're isomers of each other so we call them tautomers and they're in equilibrium with each other. It can spontaneously through equilibrium get to the actual enol form. Web identify the keto form of the enol tautomer shown below: If, however, protonation takes place on the. Identify the enol form of each keto tautomer. You'll get a detailed solution from a subject matter expert that helps you learn. 1) protonation of the carbonyl. Web in a solution, you won't see much of the enol form, but these can occur. Web which of the following have an enol form?i. You'll get a detailed solution from a subject matter expert that helps you learn. The relative stability of these two. The preferred enol tautomer form can be. Web if protonation of the enolate ion takes place on the α carbon, the keto tautomer is regenerated and no net change occurs. Web identify the keto form of the enol tautomer shown below: Acids and bases both bring about the. Web in a solution, you won't see much of the enol form,. A b c d this problem has been solved! Web if protonation of the enolate ion takes place on the α carbon, the keto tautomer is regenerated and no net change occurs. They're isomers of each other so we call them tautomers and they're in equilibrium with each other. It can spontaneously through equilibrium get to the actual enol form.. This problem has been solved! Identify the enol form of each keto tautomer. Acids and bases both bring about the. Web alkehydes and symmetrical ketones typically only have one possible enol tautomer while asymmetrical ketones have two or more. And so you could imagine,. At the same time, we need to remove the hydroxyl group (oh) from the carbon and replace it with a hydrogen atom (h). If, however, protonation takes place on the. The relative stability of these two. Web identify the keto form of the enol tautomer shown below: You'll get a detailed solution from a subject matter expert that helps you learn. A b c d this problem has been solved! The preferred enol tautomer form can be. Web in a solution, you won't see much of the enol form, but these can occur. You'll get a detailed solution from a subject matter expert. Web which of the following have an enol form?i. It can spontaneously through equilibrium get to the actual enol form. 1) protonation of the carbonyl. Tautomers are readily interconverted constitutional isomers, usually distinguished by a different location for an atom or a group. Web if protonation of the enolate ion takes place on the α carbon, the keto tautomer is regenerated and no net change occurs. Web the keto form and the enol form, and these are different molecules.Keto Enol Tautomerism A Summary of Key Points — Master Organic Chemistry
Ketoenol tautomerism reaction Royalty Free Vector Image
[Solved] What is the predominant enol tautomer of 9to5Science
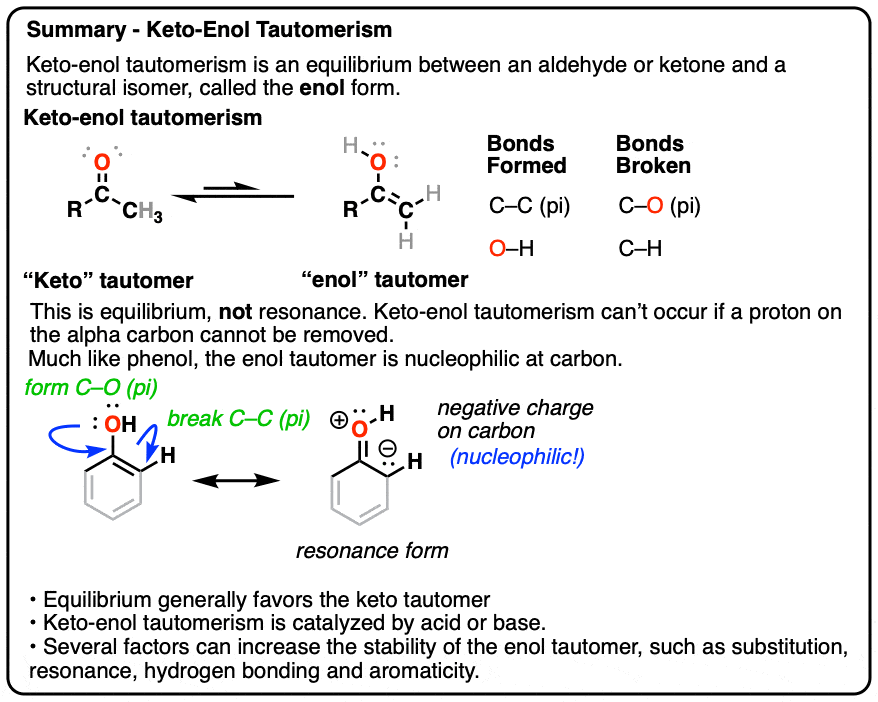
KetoEnol Tautomerism Key Points Master Organic Chemistry
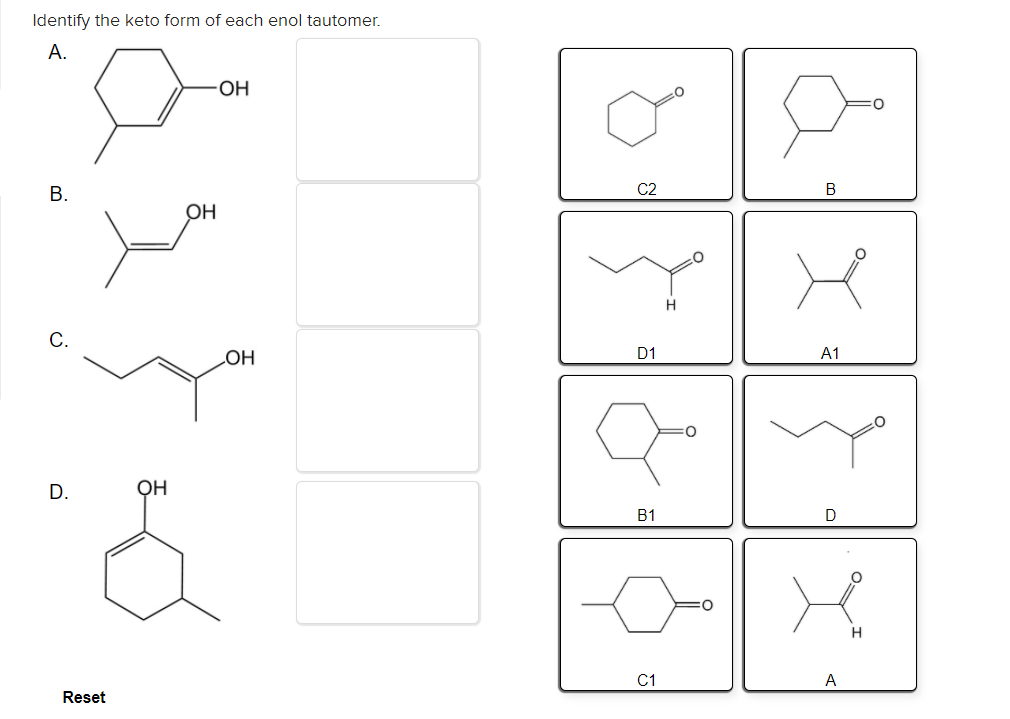
Solved Identify the keto form of each enol tautomer. А. ОН
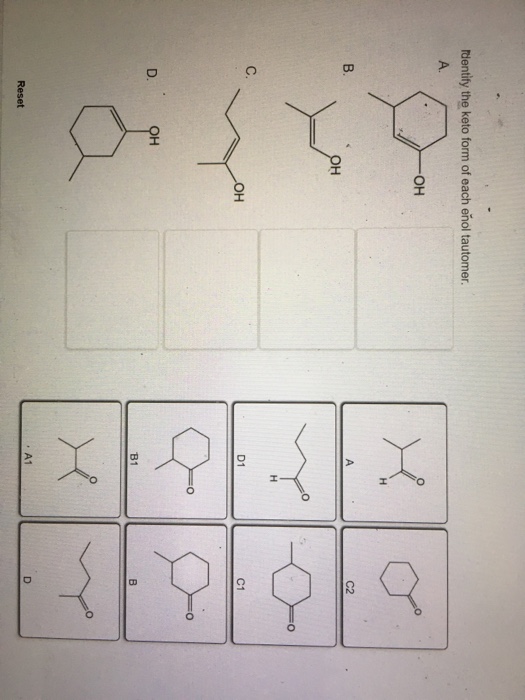
Solved dentify the keto form of each enol tautomer он C2 C.
Keto Enol Tautomerism What Is It and Why Is It Important?
Solved Identify the enol form of each keto tautomer.
Keto Enol Tautomerization Reaction and Mechanism in Acid and Base
Keto Enol Tautomerism A Summary of Key Points — Master Organic Chemistry
Related Post:

![[Solved] What is the predominant enol tautomer of 9to5Science](https://i.stack.imgur.com/FsOH2.jpg)