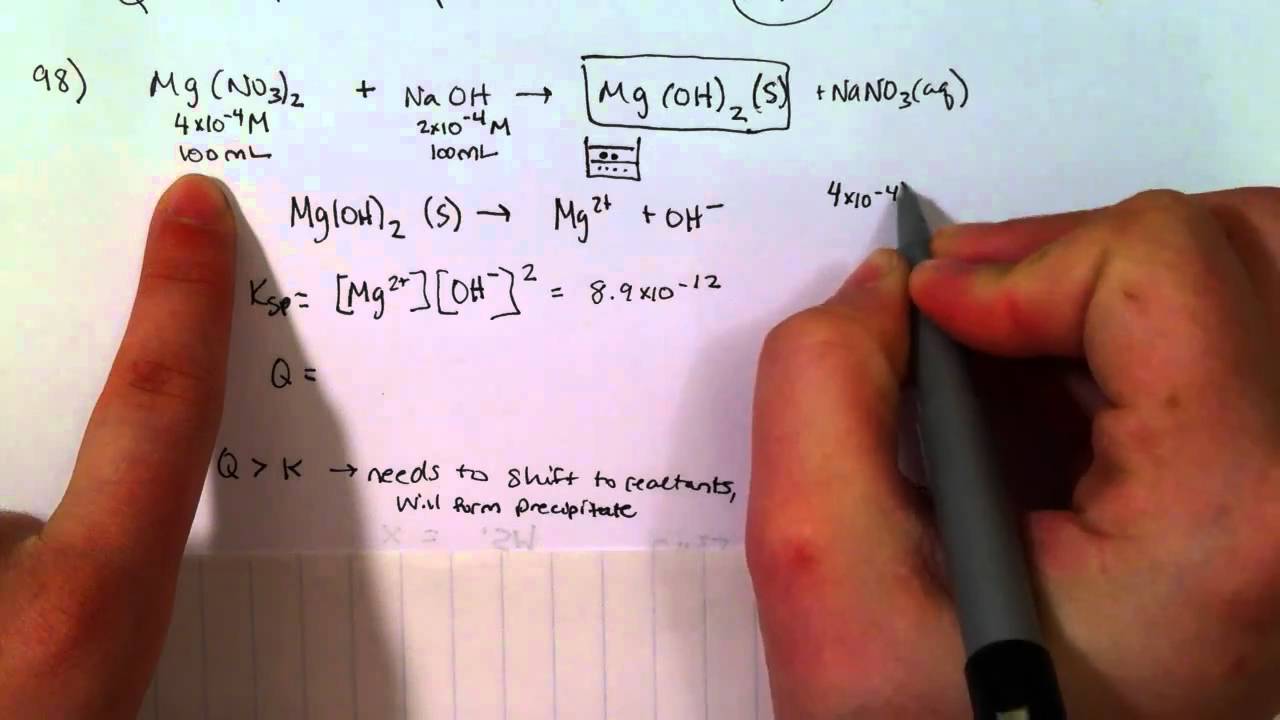
How To Determine If A Precipitate Will Form
How To Determine If A Precipitate Will Form - Web we would like to show you a description here but the site won’t allow us. Web 🎯 want to ace chemistry? Web predicting precipitates knowledge of ksp values will allow you to be able to predict whether or not a precipitate will form when two solutions are mixed together. A precipitation reaction will always have a solid. Web real chemistry 10.6k subscribers subscribe 17k views 7 years ago chapter 4 of principles of chemistry by tro in this video you will learn how to predict the products of a precipitation. Web a double replacement reaction is specifically classified as a precipitation reaction when the chemical equation in question occurs in aqueous solution and one. The formation of the precipitate lowers the concentration of each. Web to determine whether a precipitate will form, we must calculate the ionic concentrations at the moment of mixing. Web if q > ksp, a precipitate will form. Web if the value of the ion product is greater than the value of the \(k_\text{sp}\), then a precipitate will form. Web up to 6% cash back now we will calculate the theoretical yield of each reactant. So it's exceeded the limit of what can dissolve, and therefore you can imagine some lead two plus ions combining with. The formation of the precipitate lowers the concentration of each. Note that precipitation may not happen immediately if q is equal to or. Read through the given information in the problem for the chemical reaction. Web predicting precipitates knowledge of ksp values will allow you to be able to predict whether or not a precipitate will form when two solutions are mixed together. The limiting reagent is and this reaction. Determine if two aqueous compounds are on the. Web if the value of. This was a simple calculation mistake.the problem setup is still correct. Web to determine whether a precipitate will form, we must calculate the ionic concentrations at the moment of mixing. Web up to 6% cash back the precipitate will be the product that is insoluble in water, so your choices are or. Web a substance will precipitate when solution conditions. Web real chemistry 10.6k subscribers subscribe 17k views 7 years ago chapter 4 of principles of chemistry by tro in this video you will learn how to predict the products of a precipitation. Calculate the initial concentrations the. Nitrate salts are always soluble in water, as are alkali metal salts such as. Web a double replacement reaction is specifically classified. Ammonium (nh 4+) compounds are soluble. Web we can use the reaction quotient to predict whether a precipitate will form when two solutions containing dissolved ionic compounds are mixed. Web up to 6% cash back now we will calculate the theoretical yield of each reactant. Web to determine whether a precipitate will form, we must calculate the ionic concentrations at. Web if q > ksp, a precipitate will form. So it's exceeded the limit of what can dissolve, and therefore you can imagine some lead two plus ions combining with. Read through the given information in the problem for the chemical reaction. A solution could be supersaturated for. A precipitation reaction will always have a solid. Nitrate salts are always soluble in water, as are alkali metal salts such as. How to identify a precipitation reaction. Web real chemistry 10.6k subscribers subscribe 17k views 7 years ago chapter 4 of principles of chemistry by tro in this video you will learn how to predict the products of a precipitation. Ammonium (nh 4+) compounds are soluble. Two. The formation of the precipitate lowers the concentration of each. Identify the reactants and products. Web if the value of the ion product is greater than the value of the \(k_\text{sp}\), then a precipitate will form. Determine if two aqueous compounds are on the. So it's exceeded the limit of what can dissolve, and therefore you can imagine some lead. Identify the reactants and products. Web these reactions are commonly used to help determine what ions are in the solution. Now we perform the same calculation beginning with : Web we would like to show you a description here but the site won’t allow us. Note that precipitation may not happen immediately if q is equal to or greater than. Substances with relatively low solubilities are said to be insoluble ,. Determine if two aqueous compounds are on the. Web these reactions are commonly used to help determine what ions are in the solution. Note that precipitation may not happen immediately if q is equal to or greater than ksp. This was a simple calculation mistake.the problem setup is still. Web we would like to show you a description here but the site won’t allow us. Web up to 6% cash back now we will calculate the theoretical yield of each reactant. Web predicting precipitates knowledge of ksp values will allow you to be able to predict whether or not a precipitate will form when two solutions are mixed together. So it's exceeded the limit of what can dissolve, and therefore you can imagine some lead two plus ions combining with. Note that precipitation may not happen immediately if q is equal to or greater than ksp. Substances with relatively low solubilities are said to be insoluble ,. The molarity of cacl2 should be.00100 m instead of 0.00050 m. Web when qsp is greater than ksp, the solution is oversaturated. Nitrate salts are always soluble in water, as are alkali metal salts such as. Identify the reactants and products. The formation of the precipitate lowers the concentration of each. A solution could be supersaturated for. Two aqueous solutions can mix and the ions inside can. Web real chemistry 10.6k subscribers subscribe 17k views 7 years ago chapter 4 of principles of chemistry by tro in this video you will learn how to predict the products of a precipitation. Web a double replacement reaction is specifically classified as a precipitation reaction when the chemical equation in question occurs in aqueous solution and one. If q is less than kₛₚ,. Web if q > ksp, a precipitate will form. Calculate the initial concentrations the. How to identify a precipitation reaction. Read through the given information in the problem for the chemical reaction.How to Determine if Precipitate will Form or Not Examples, Practice
Complete the reaction and determine if a precipitate forms 4 YouTube
PPT Ch. 13 Precipitation Reactions PowerPoint Presentation, free
PPT Precipitate Reactions PowerPoint Presentation ID3197585
12 use Ksp to find if a precipitate will form YouTube
SOLVEDCalculate whether a precipitate will form
Precipitate Definition and Example in Chemistry
Predicting formation of a precipitate YouTube
Example Determining Whether a Precipitate Will Form (Solubility
Q Will a Precipitate Form (Z.15.97) YouTube
Related Post:



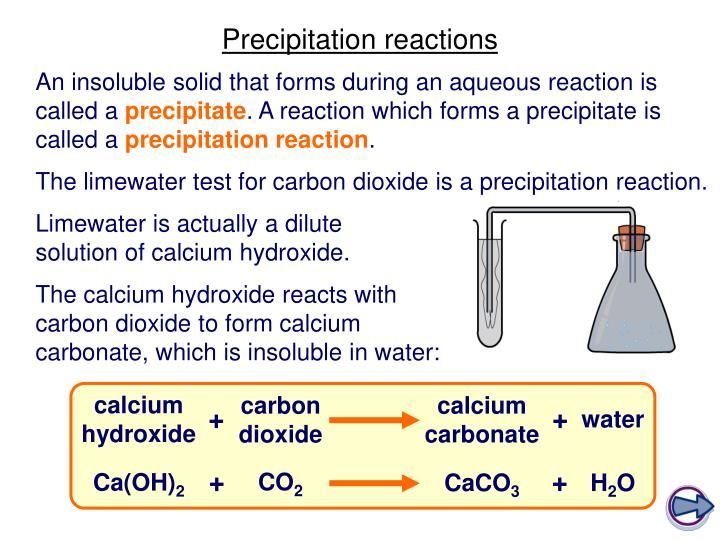


:max_bytes(150000):strip_icc()/precipitate-589cb8953df78c47581a9014.jpg)