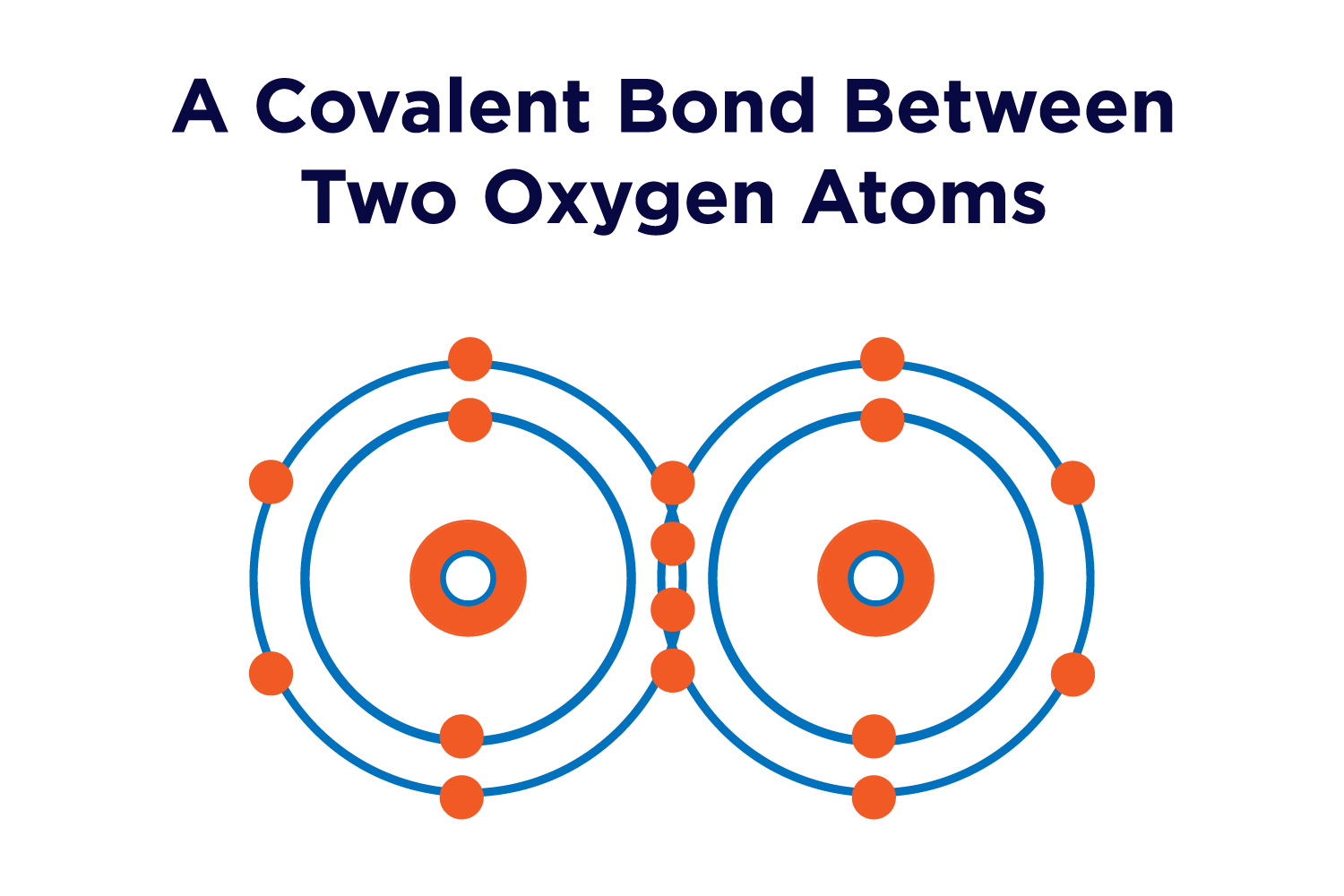
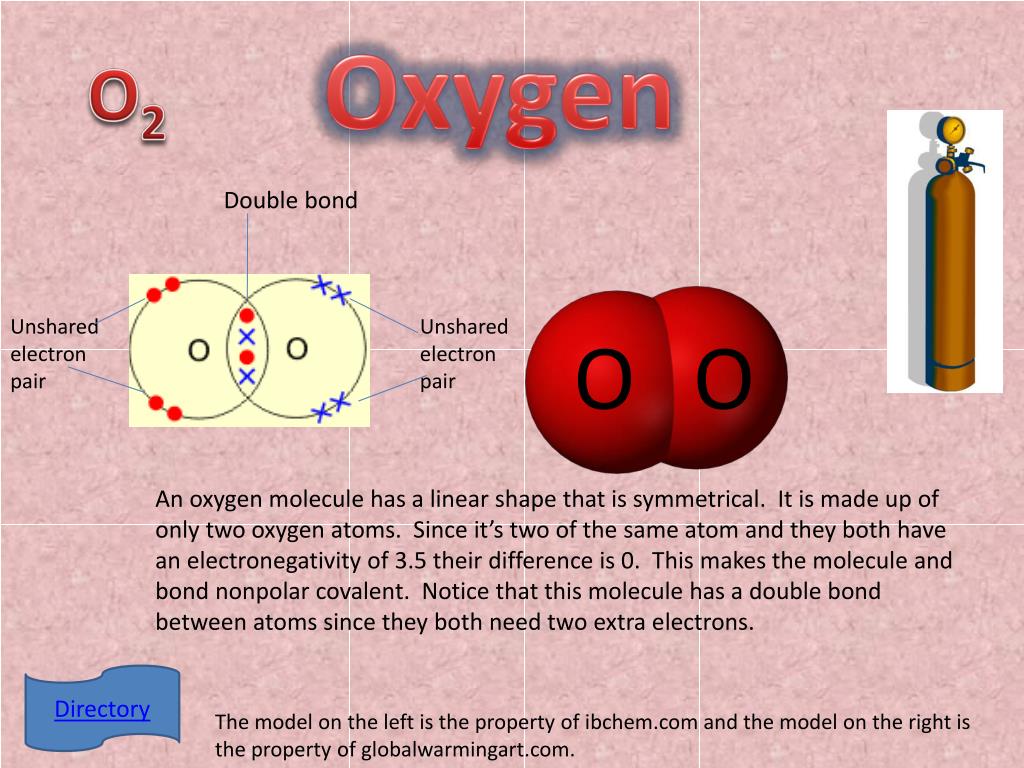
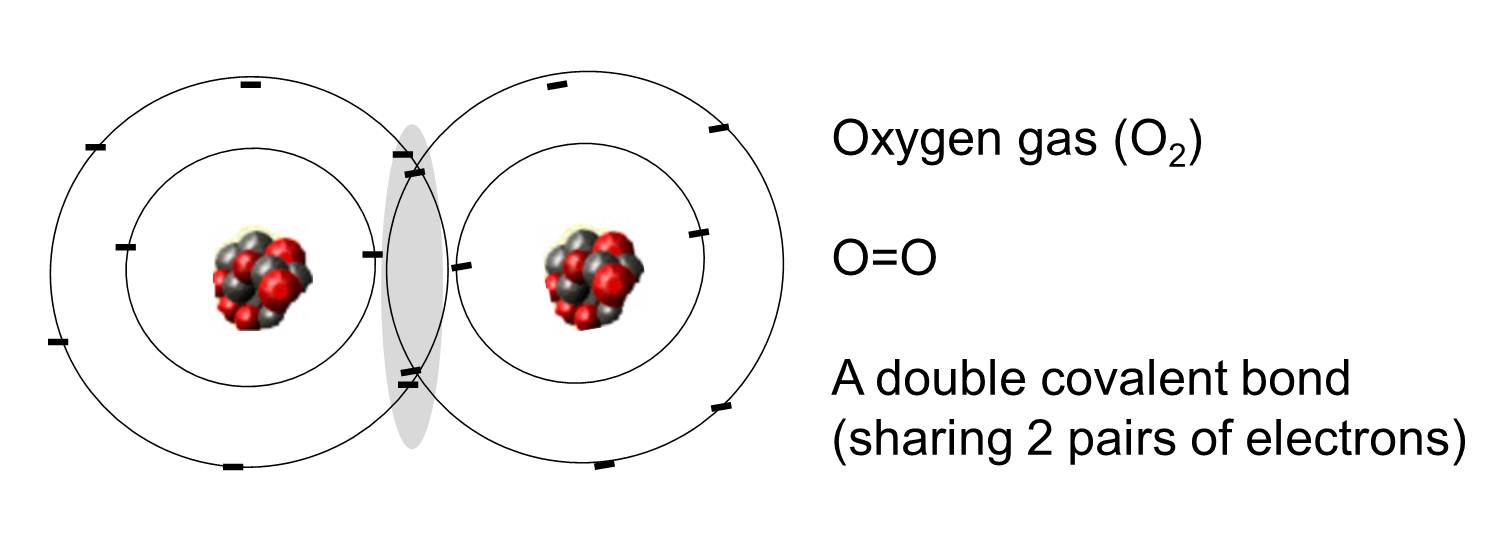
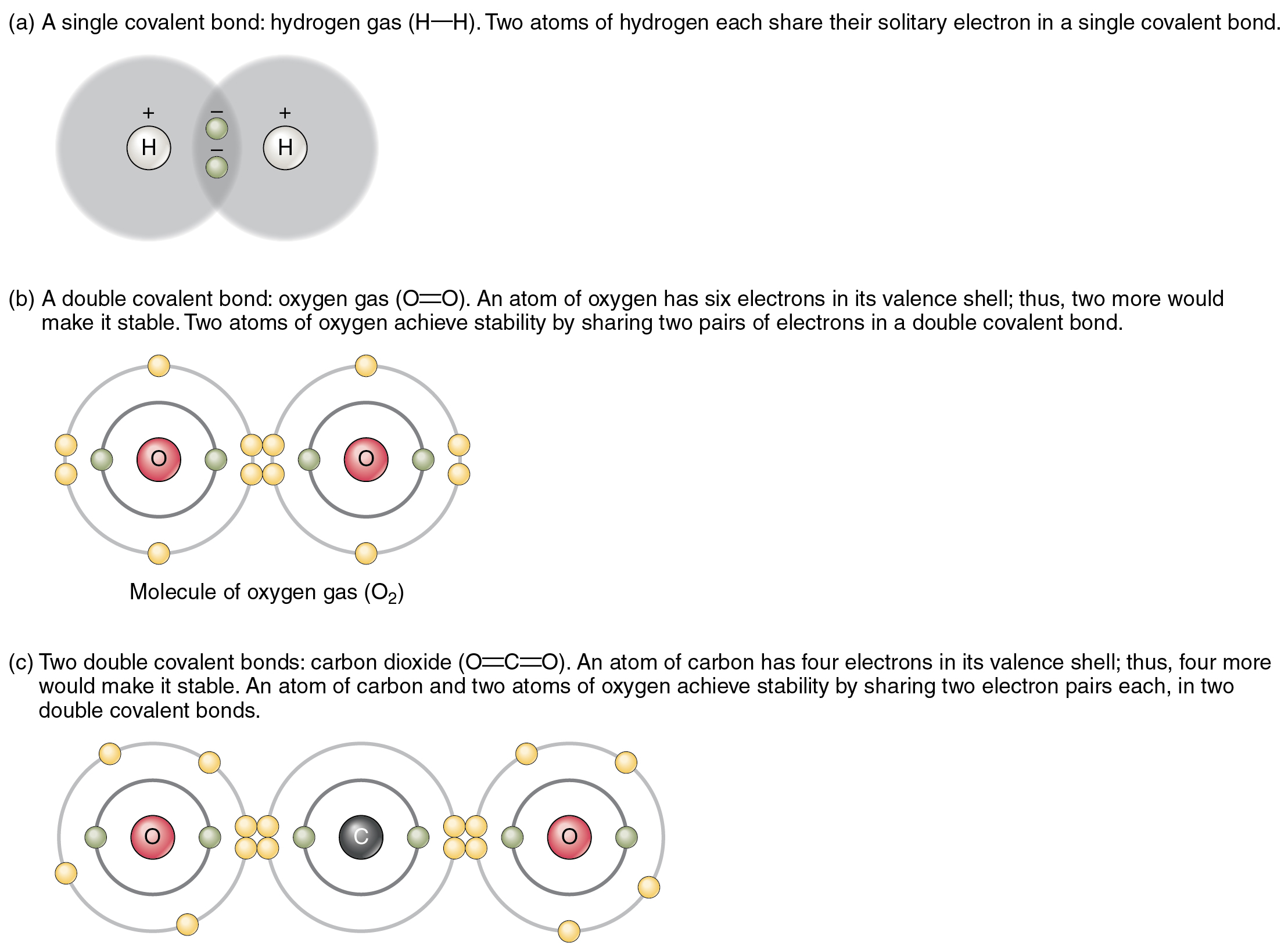
How Many Covalent Bonds Does Oxygen Form
How Many Covalent Bonds Does Oxygen Form - You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Oxygen and other atoms in group 6a (16) obtain an octet by forming two. Therefore the maximum number of covalent bonds. Web this problem has been solved! Oxygen and other atoms in group 6a (16) obtain an octet by forming two covalent. Web the number refers to the number of bonds each of the element makes: Web oxygen and other atoms in group 6a (16) obtain an octet by forming two covalent bonds. It is easier for an oxygen atom to accept or share two electrons instead of. Web atoms of different elements close element a substance made of one type of atom only. The number of electrons required to obtain an octet determines the number of covalent. How many covalent bonds does carbon form if each of its unpaired electrons participate in one bond? Web oxygen can form two single bonds because it has six valent electrons on its outer shell. Web the number refers to the number of bonds each of the element makes: Web meallic elements can definiely have more than eight valence electrons, however. Web to obtain an octet, these atoms form three covalent bonds, as in nh 3 (ammonia). Is determined by the distance at which the lowest potential energy is achieved. Web group 5a elements such as nitrogen have five valence electrons in the atomic lewis symbol: Web oxygen and other atoms in group 6a (16) obtain an octet by forming two. Oxygen forms two single covalent bonds, carbon forms four single covalent bonds and hydrogen forms one single covalent bond. Web atoms of different elements close element a substance made of one type of atom only. One electron is provided by each atom, and the pair of electrons is. Web group 5a elements such as nitrogen have five valence electrons in. How many covalent bonds does carbon form if each of its unpaired electrons participate in one bond? Web a covalent bond is the force of attraction that holds together two nonmetal atoms that share a pair of electrons. The potential energy of two separate hydrogen atoms (right) decreases as. You'll get a detailed solution from a subject matter expert that. How many covalent bonds does carbon form if each of its unpaired electrons participate in one bond? These four elements are widely used when it comes to drawing lewis structures at. Web oxygen can form two single bonds because it has six valent electrons on its outer shell. Web group 5a elements such as nitrogen have five valence electrons in. Web this problem has been solved! Will form either one, two, three or four covalent bonds with other atoms. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web science chemistry chemistry questions and answers 1. Alone, each oxygen atom of group 6a or group 16 has six valence electrons. It is easier for an oxygen atom to accept or share two electrons instead of. Covalent bonds form because they give atoms a more stable arrangement of electrons. Oxygen forms two single covalent bonds, carbon forms four single covalent bonds and hydrogen forms one single covalent bond. Is determined by the distance at which the lowest potential energy is achieved.. Alone, each oxygen atom of group 6a or group 16 has six valence electrons. The potential energy of two separate hydrogen atoms (right) decreases as. One lone pair and three unpaired electrons. Web group 5a elements such as nitrogen have five valence electrons in the atomic lewis symbol: The number of electrons required to obtain an octet determines the number. Alone, each oxygen atom of group 6a or group 16 has six valence electrons. To obtain an octet, these atoms. How many covalent bonds does carbon form if each of its unpaired electrons participate in one bond? Web this problem has been solved! Will form either one, two, three or four covalent bonds with other atoms. The number of electrons required to obtain an octet determines the number of covalent. Therefore the maximum number of covalent bonds. Oxygen and other atoms in group 6a (16) obtain an octet by forming two covalent. One lone pair and three unpaired electrons. Alone, each oxygen atom of group 6a or group 16 has six valence electrons. Web oxygen and other atoms in group 16 obtain an octet by forming two covalent bonds: Web group 5a elements such as nitrogen have five valence electrons in the atomic lewis symbol: Therefore the maximum number of covalent bonds. These four elements are widely used when it comes to drawing lewis structures at. How many covalent bonds does carbon form if each of its unpaired electrons participate in one bond? Oxygen and other atoms in group 6a (16) obtain an octet by forming two. Oxygen forms two single covalent bonds, carbon forms four single covalent bonds and hydrogen forms one single covalent bond. Web meallic elements can definiely have more than eight valence electrons, however they do not tend to form covalent bonds. Web oxygen can form two single bonds because it has six valent electrons on its outer shell. Web the number refers to the number of bonds each of the element makes: The potential energy of two separate hydrogen atoms (right) decreases as. Alone, each oxygen atom of group 6a or group 16 has six valence electrons. Will form either one, two, three or four covalent bonds with other atoms. Covalent bonds form because they give atoms a more stable arrangement of electrons. Web atoms of different elements close element a substance made of one type of atom only. Hydrogen makes 1 bond, oxygen makes 2 bonds, nitrogen makes 3 bonds and carbon makes 4 bonds. Is determined by the distance at which the lowest potential energy is achieved. Web a covalent bond is the force of attraction that holds together two nonmetal atoms that share a pair of electrons. Oxygen and other atoms in group 6a (16) obtain an octet by forming two covalent. Fluorine and the other halogens in group 7a (17) have seven valence.Nonpolar Covalent Bond Definition and Examples
PPT Covalent Bonds PowerPoint Presentation, free download ID2367962
Basic Cell Biology
The top panel in this figure shows two hydrogen atoms sharing two
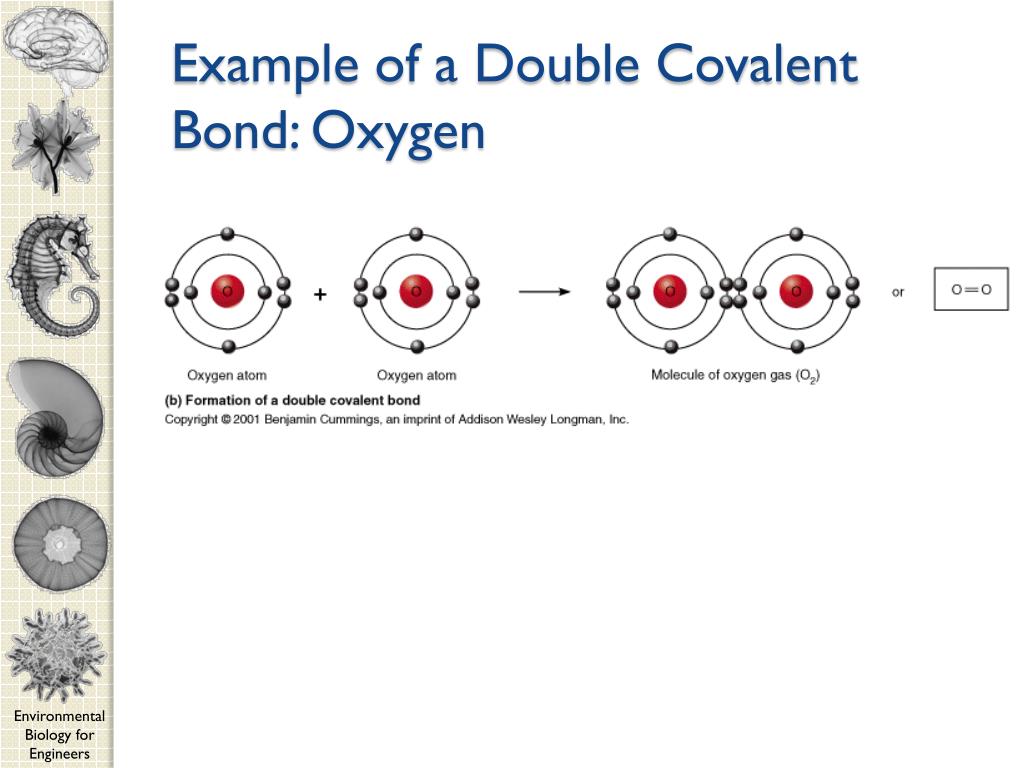
PPT CEE 210 Environmental Biology for Engineers PowerPoint
PPT Covalent Bonds PowerPoint Presentation, free download ID6647183
covalent bond Definition, Properties, Examples, & Facts Britannica
Covalent bonding in an oxygen molecule.
Covalent Bond Biology Dictionary
Oxygen Covalent Bond Diagram
Related Post: