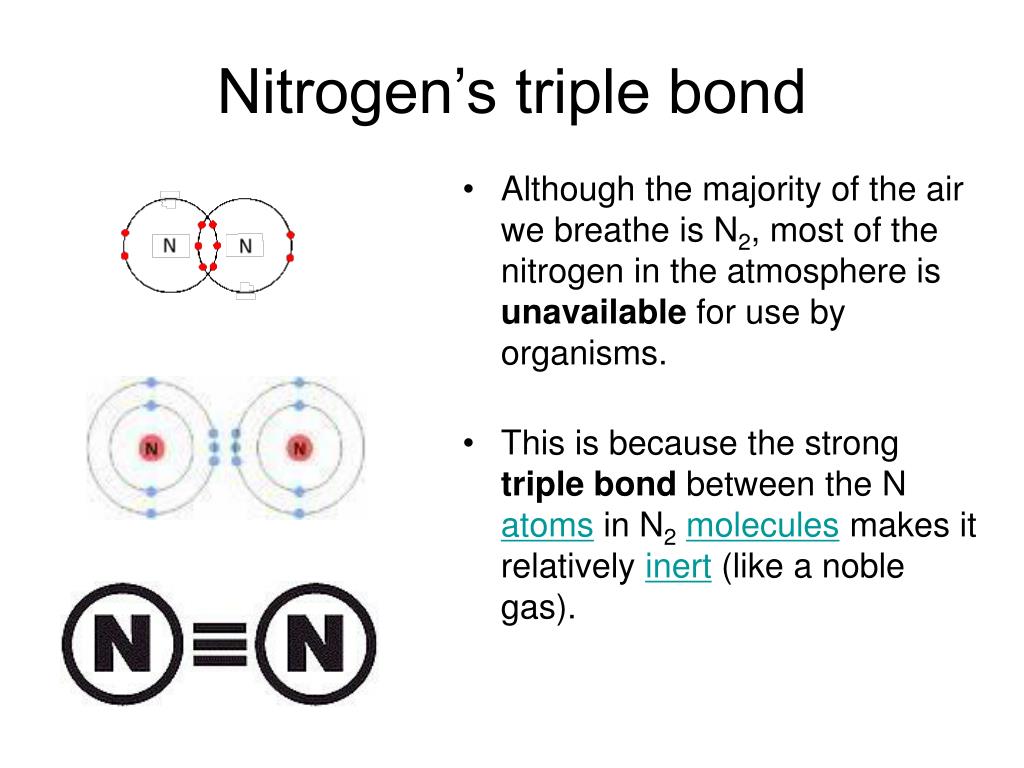
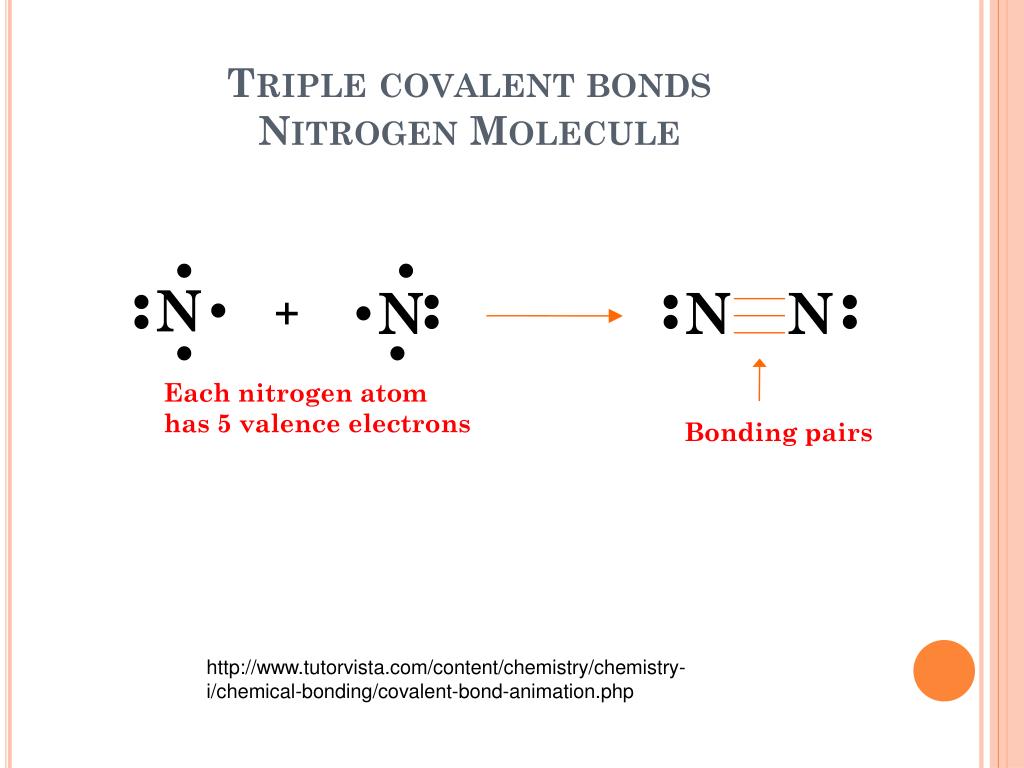
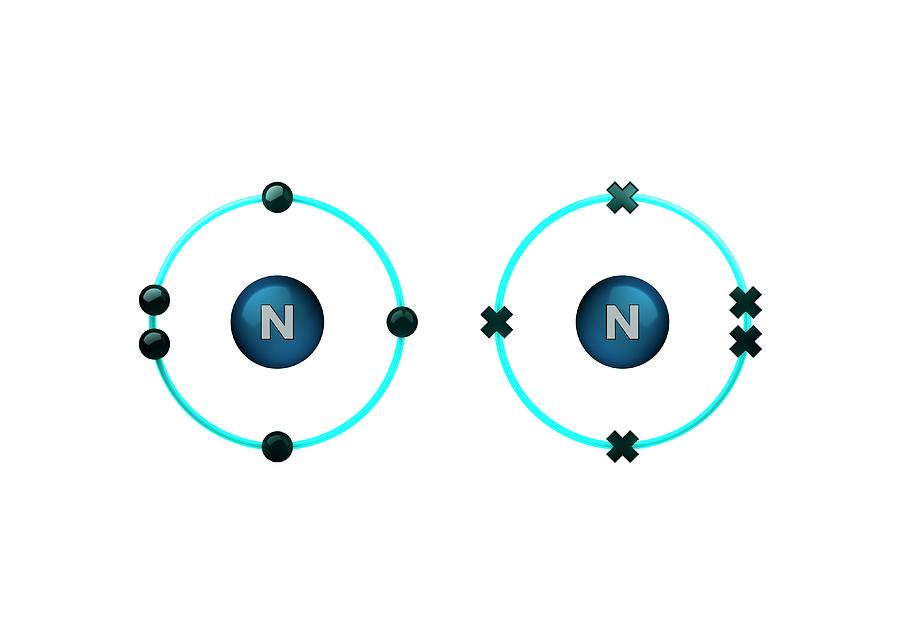
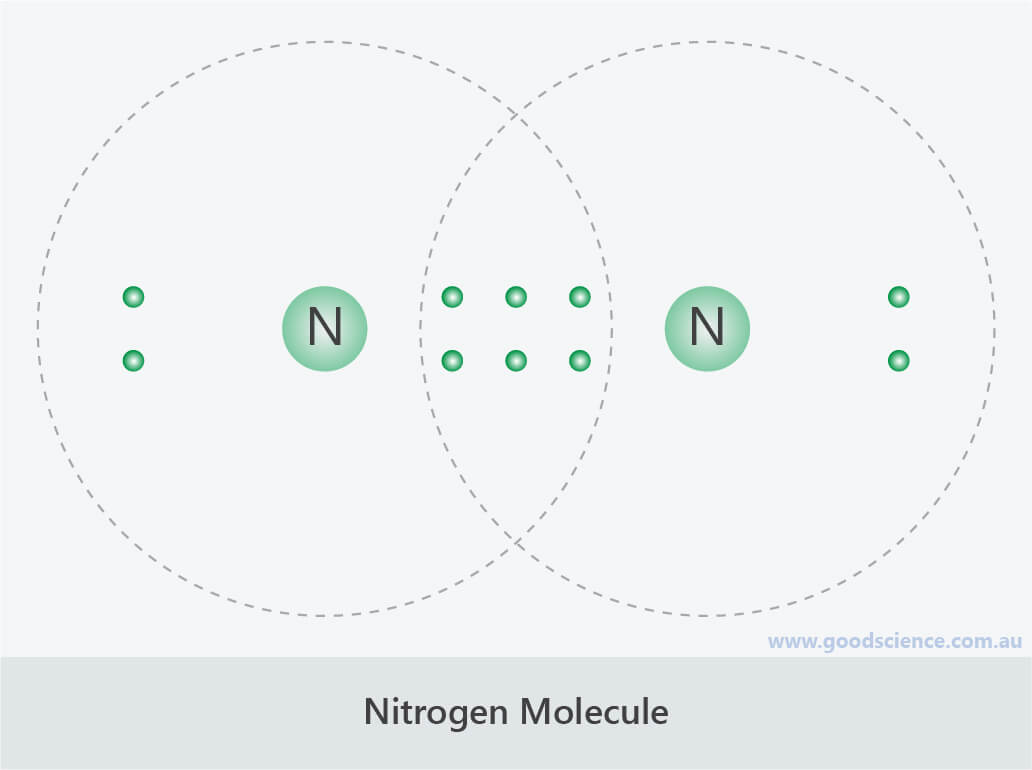
How Many Covalent Bonds Does Nitrogen Form
How Many Covalent Bonds Does Nitrogen Form - How many covalent bonds will a nitrogen atom usually form? These are normally formed with other metals and look like: Web how many covalent bonds does carbon generally form in organic compounds? Hydrogen makes 1 bond, oxygen. A) 2 b) 3 c) 4 d) 8 b which element is not a common heteroatom in organic compounds? Thus, bonding in potassium nitrate is ionic, resulting from the electrostatic. Oxygen and other atoms in. One lone pair and three unpaired electrons. Through that pair, nitrogen can form an. Web for example, potassium nitrate, kno 3, contains the k + cation and the polyatomic no 3 − anion. A) 2 b) 3 c) 4 d) 8 b which element is not a common heteroatom in organic compounds? Group 5a (15) elements such as nitrogen. The bond that is formed by the mutual sharing of electrons. The formal charge on the bromine atom in bro3 drawn with three single bonds. How many covalent bonds will a nitrogen atom usually. Oxygen and other atoms in. Web nitrogen has five valence electrons and in simple amines it is trivalent, with the two remaining electrons forming a lone pair. The electrons involved are in. Web for example, potassium nitrate, kno 3, contains the k + cation and the polyatomic no 3 − anion. One lone pair and three unpaired electrons. These are normally formed with other metals and look like: Sap‑3 (eu) , sap‑3.a (lo) , sap‑3.a.2 (ek) , sap‑3.a.3 (ek) google. Web for example, potassium nitrate, kno 3, contains the k + cation and the polyatomic no 3 − anion. Nitrogen typically forms 3 covalent bonds, including in n 2 this is because it has atomic number 7 ,. A) 2 b) 3 c) 4 d) 8 b which element is not a common heteroatom in organic compounds? To obtain an octet, these atoms. Web these four electrons can be gained by forming four covalent bonds, as illustrated here for carbon in ch 4 (methane). These are normally formed with other metals and look like: The number refers to. Oxygen and other atoms in. One lone pair and three unpaired electrons. Web nitrogen has five valence electrons and in simple amines it is trivalent, with the two remaining electrons forming a lone pair. The number refers to the number of bonds each of the element makes: Sap‑3 (eu) , sap‑3.a (lo) , sap‑3.a.2 (ek) , sap‑3.a.3 (ek) google. Web nitrogen has five valence electrons and in simple amines it is trivalent, with the two remaining electrons forming a lone pair. Nitrogen typically forms 3 covalent bonds, including in n 2 this is because it has atomic number 7 , so its electron configuration is 1s 22s 22p 3 giving it 5 valence shell. Web introduction to biological macromolecules. Web introduction to biological macromolecules © 2023 khan academy covalent bonds ap.chem: Web are formed by nitrogen if each of its unpaired electrons participates in one bond. Web two different atoms can also share electrons and form covalent bonds. Group 5a (15) elements such as nitrogen. Hydrogen makes 1 bond, oxygen. Web for example, potassium nitrate, kno 3, contains the k + cation and the polyatomic no 3 − anion. Is determined by the distance at which the lowest potential energy is achieved. Through that pair, nitrogen can form an. The number refers to the number of bonds each of the element makes: One lone pair and three unpaired electrons. Nitrogen typically forms 3 covalent bonds, including in n 2 this is because it has atomic number 7 , so its electron configuration is 1s 22s 22p 3 giving it 5 valence shell. The electrons involved are in. One lone pair and three unpaired electrons. For example, water, (\(\ce{h2o}\)), has two covalent bonds between a single oxygen atom and two.. Web when nitrogen forms compounds with other atoms, it primarily forms covalent bonds. One lone pair and three unpaired electrons. Is determined by the distance at which the lowest potential energy is achieved. A) 2 b) 3 c) 4 d) 8 b which element is not a common heteroatom in organic compounds? Group 5a (15) elements such as nitrogen. A) 2 b) 3 c) 4 d) 8 b which element is not a common heteroatom in organic compounds? Web two different atoms can also share electrons and form covalent bonds. To obtain an octet, these atoms. One lone pair and three unpaired electrons. Group 5a (15) elements such as nitrogen. Web it’s called the honc rule, or sometimes known as honc 1234 rule. These are normally formed with other metals and look like: The formal charge on the bromine atom in bro3 drawn with three single bonds. Thus, bonding in potassium nitrate is ionic, resulting from the electrostatic. Ad browse & discover thousands of science book titles, for less. Sap‑3 (eu) , sap‑3.a (lo) , sap‑3.a.2 (ek) , sap‑3.a.3 (ek) google. Web these four electrons can be gained by forming four covalent bonds, as illustrated here for carbon in ch 4 (methane). Oxygen and other atoms in. Web introduction to biological macromolecules © 2023 khan academy covalent bonds ap.chem: One lone pair and three unpaired electrons. Web group 5a (15) elements such as nitrogen have five valence electrons in the atomic lewis symbol: Web how many covalent bonds does carbon generally form in organic compounds? The potential energy of two separate hydrogen atoms (right) decreases as. Nitrogen typically forms 3 covalent bonds, including in n 2 this is because it has atomic number 7 , so its electron configuration is 1s 22s 22p 3 giving it 5 valence shell. Web for example, potassium nitrate, kno 3, contains the k + cation and the polyatomic no 3 − anion.PPT The Nitrogen Cycle PowerPoint Presentation, free download ID899587
Case Based MCQ Chemistry in Automobiles For an internal combustion
Covalent Bond Biology Dictionary
How do the Nitrogen atoms combine&n
How to find Valency? What are valence electrons? Teachoo
How many bonds does Nitrogen form? YouTube
PPT COVALENT BONDING PowerPoint Presentation, free download ID5128236
Bond Formation In Nitrogen Molecule Photograph by
PPT Covalent bonding PowerPoint Presentation, free download ID9726629
Ionic and Covalent Compounds Good Science
Related Post: