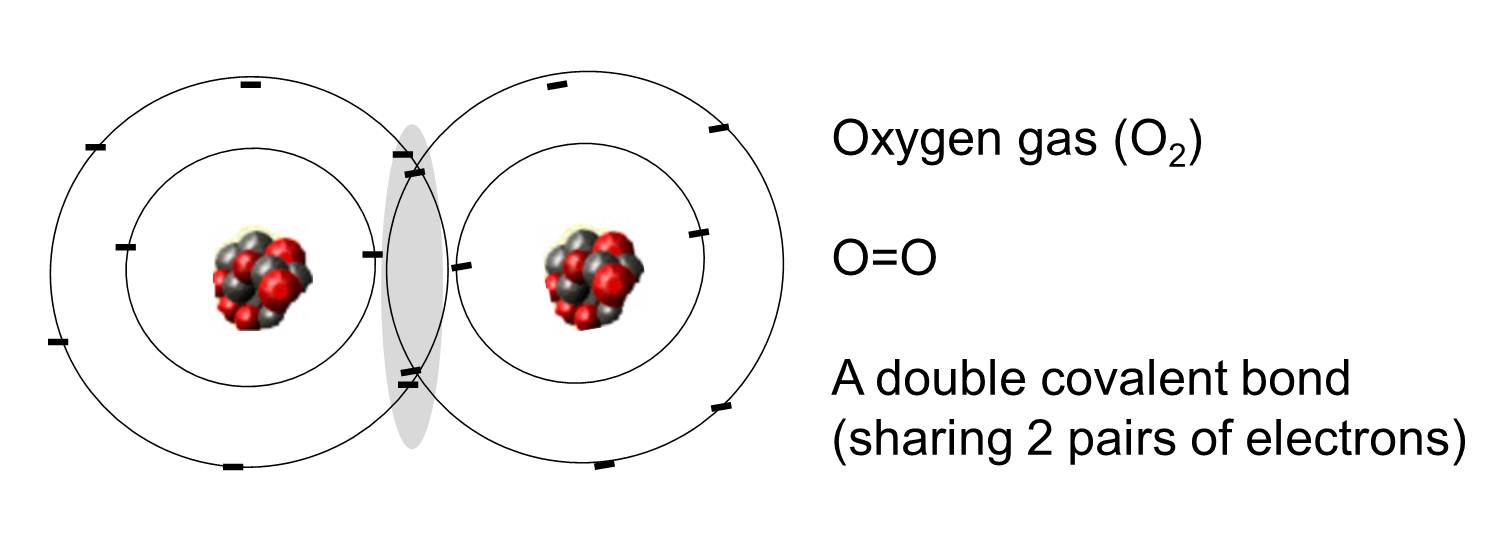
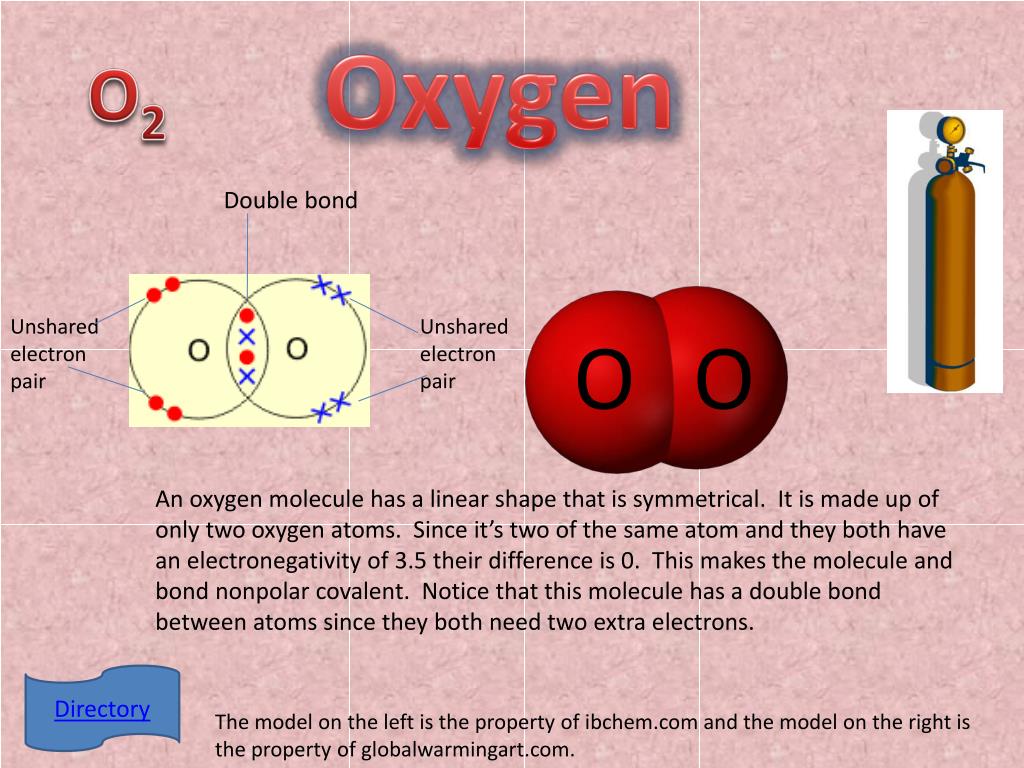
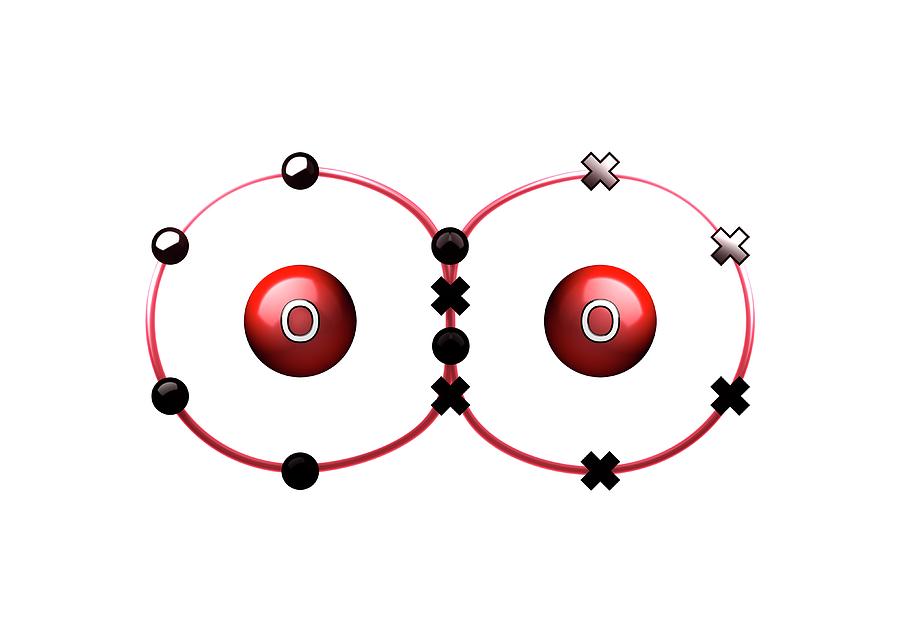
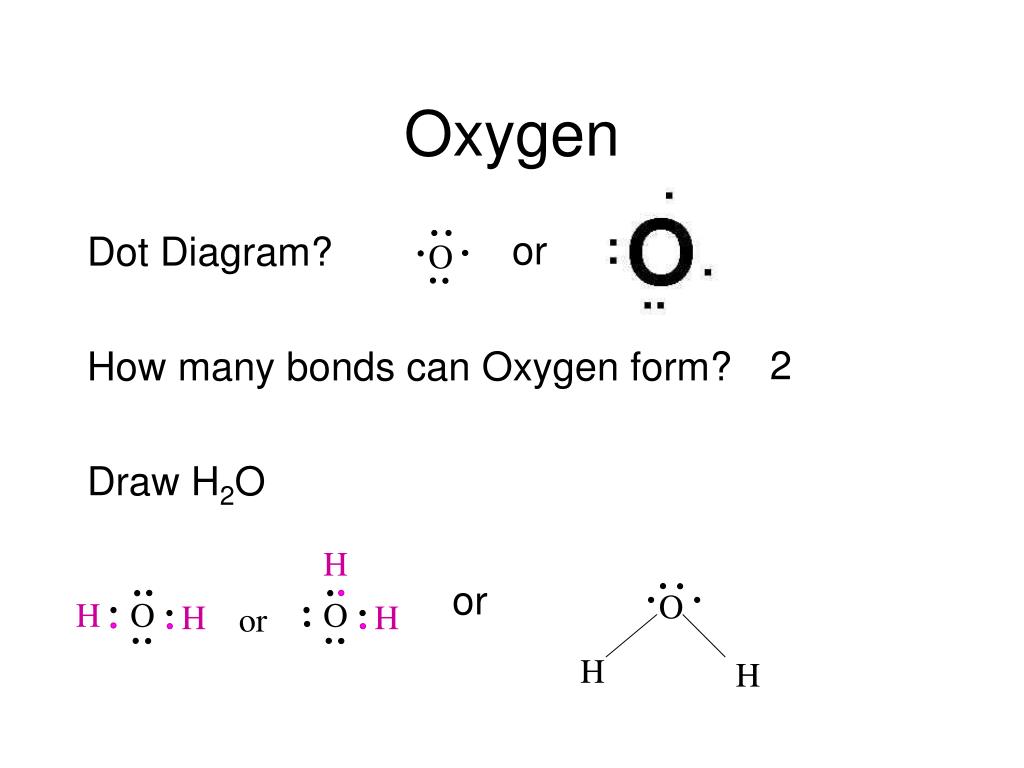
How Many Bonds Does Oxygen Form
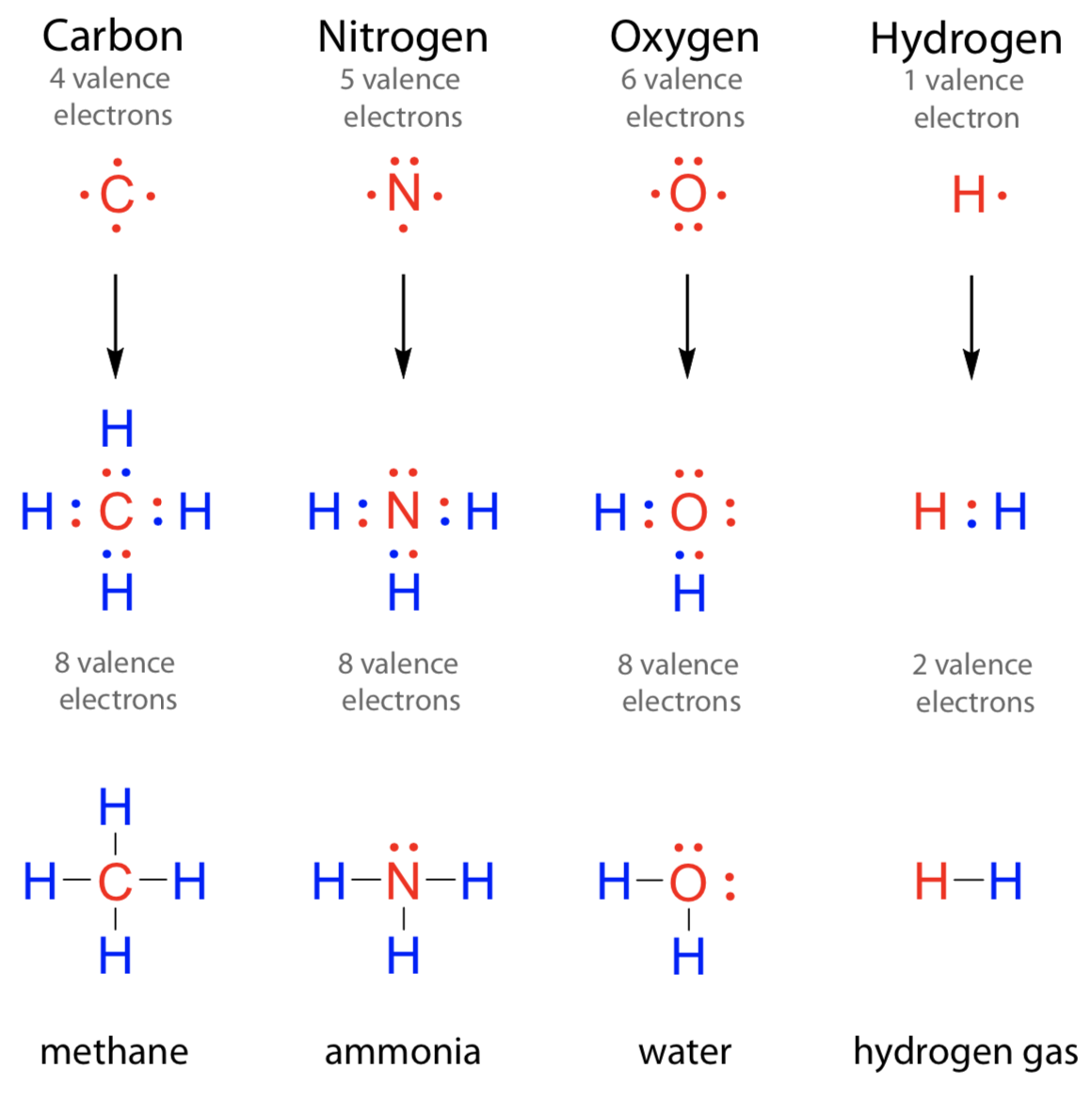
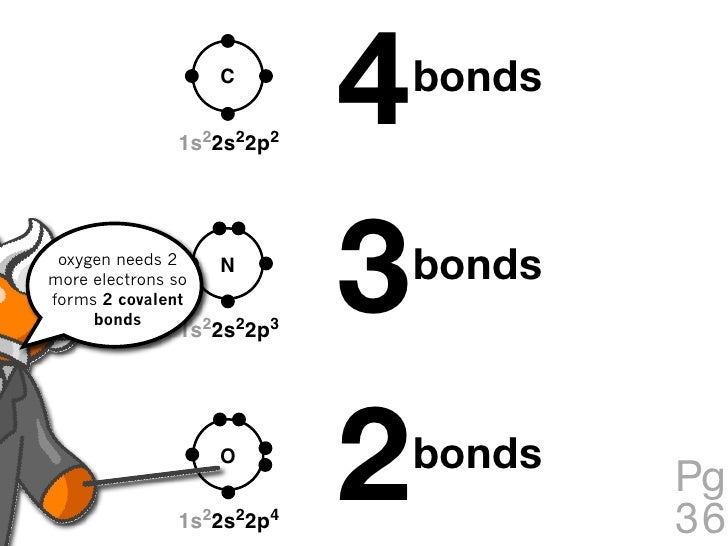
How Many Bonds Does Oxygen Form - It consists of two hydrogen (h) atoms and one. Web to obtain an octet, these atoms form three covalent bonds, as in nh 3 (ammonia). They become a cl 2 molecule. Two core and seven valence. Does this make sense based on the number of valence electrons in an oxygen atom? Web to obtain an octet, these atoms form three covalent bonds, as in nh 3 (ammonia). By sharing two pairs of valence electrons, each oxygen atom has a total of eight valence electrons. Using the same explanation for the rest will help explain how elements form the given number of bonds: Ad browse & discover thousands of science book titles, for less. Web that is, the oxygen is sp 3 hybridized with a tetrahedral electronic geometry, having two bonding orbitals and two lone pairs. Web the first oxygen has three bonds, the second only has one. Group 5a elements form 3 bonds. Group 4a elements form 4 bonds. Oxygen forms two single covalent bonds, carbon forms four single covalent bonds and hydrogen forms one single covalent bond. Web to obtain an octet, these atoms form three covalent bonds, as in nh 3 (ammonia). It consists of two hydrogen (h) atoms and one. Web to obtain an octet, these atoms form three covalent bonds, as in nh 3 (ammonia). Two core and seven valence. To understand why chemical bonds form, consider the common compound known as water, or h 2 o. Group 4a elements form 4 bonds. Oxygen forms two single covalent bonds, carbon forms four single covalent bonds and hydrogen forms one single covalent bond. Web to obtain an octet, these atoms form three covalent bonds, as in nh 3 (ammonia). The earth's crustal rock is. Web continuing on across the periodic table we see that fluorine is the next element after oxygen. 1 how many. Web oxygen is present as compounds in the atmosphere in trace quantities in the form of carbon dioxide ( co 2) and oxides of nitrogen (no x ). Oxygen and other atoms in group 6a (16) obtain an octet by forming two. Web to obtain an octet, these atoms form three covalent bonds, as in nh 3 (ammonia). Web as. Web alone, each oxygen atom of group 6a or group 16 has six valence electrons. Therefore the maximum number of covalent bonds should. Web two chlorine atoms can share 1 electron each to form a single covalent bond. Web we would like to show you a description here but the site won’t allow us. Oxygen and other atoms in group. Group 5a elements form 3 bonds. You can think of the reaction taking place by a lone pair on the oxygen of one water molecule ripping off. How many bonds does each carbon, oxygen, and hydrogen atom form in maltose? Oxygen and other atoms in group 6a (16) obtain an octet by forming two. By sharing two pairs of valence. Web that is, the oxygen is sp 3 hybridized with a tetrahedral electronic geometry, having two bonding orbitals and two lone pairs. Does this make sense based on the number of valence electrons in an oxygen atom? By sharing two pairs of valence electrons, each oxygen atom has a total of eight valence electrons. Group 5a elements form 3 bonds.. One lone pair and three unpaired electrons. Does this make sense based on the number of valence electrons in an oxygen atom? By sharing two pairs of valence electrons, each oxygen atom has a total of eight valence electrons. All of these are involved with hydrogen. Oxygen forms two single covalent bonds, carbon forms four single covalent bonds and hydrogen. Oxygen and other atoms in group 6a (16) obtain an octet by forming two. Web oxygen is present as compounds in the atmosphere in trace quantities in the form of carbon dioxide ( co 2) and oxides of nitrogen (no x ). Web we would like to show you a description here but the site won’t allow us. Web to. Group 5a elements form 3 bonds. Web alone, each oxygen atom of group 6a or group 16 has six valence electrons. Web we would like to show you a description here but the site won’t allow us. Group 6a elements form 2 bonds. Web two chlorine atoms can share 1 electron each to form a single covalent bond. Group 4a elements form 4 bonds. Web to obtain an octet, these atoms form three covalent bonds, as in nh 3 (ammonia). The earth's crustal rock is. Using the same explanation for the rest will help explain how elements form the given number of bonds: Web continuing on across the periodic table we see that fluorine is the next element after oxygen. Oxygen forms two single covalent bonds, carbon forms four single covalent bonds and hydrogen forms one single covalent bond. Web to obtain an octet, these atoms form three covalent bonds, as in nh 3 (ammonia). Web that is, the oxygen is sp 3 hybridized with a tetrahedral electronic geometry, having two bonding orbitals and two lone pairs. Web as a result, oxygen will make 2 bonds. Oxygen and other atoms in group 16 obtain an octet by forming two covalent bonds: To understand why chemical bonds form, consider the common compound known as water, or h 2 o. Does this make sense based on the number of valence electrons in an oxygen atom? Web meallic elements can definiely have more than eight valence electrons, however they do not tend to form covalent bonds. You can think of the reaction taking place by a lone pair on the oxygen of one water molecule ripping off. Group 6a elements form 2 bonds. By sharing two pairs of valence electrons, each oxygen atom has a total of eight valence electrons. Group 5a elements form 3 bonds. All of these are involved with hydrogen. Web the first oxygen has three bonds, the second only has one. Two core and seven valence.Basic Cell Biology
PPT Covalent Bonds PowerPoint Presentation, free download ID2367962
Bond Formation In Oxygen Molecule Photograph by
Primary and Secondary Bonds Owlcation
PPT Chemistry Bonding PowerPoint Presentation, free download ID
LabXchange
Covalent bonding in an oxygen molecule. Covalent bonding, Chemistry
Oxygen Covalent Bond Diagram
PPT Covalent bonding PowerPoint Presentation, free download ID9726629
C 1s22s22p2 4 bonds oxygen
Related Post: