How Many Bonds Does Bromine Form
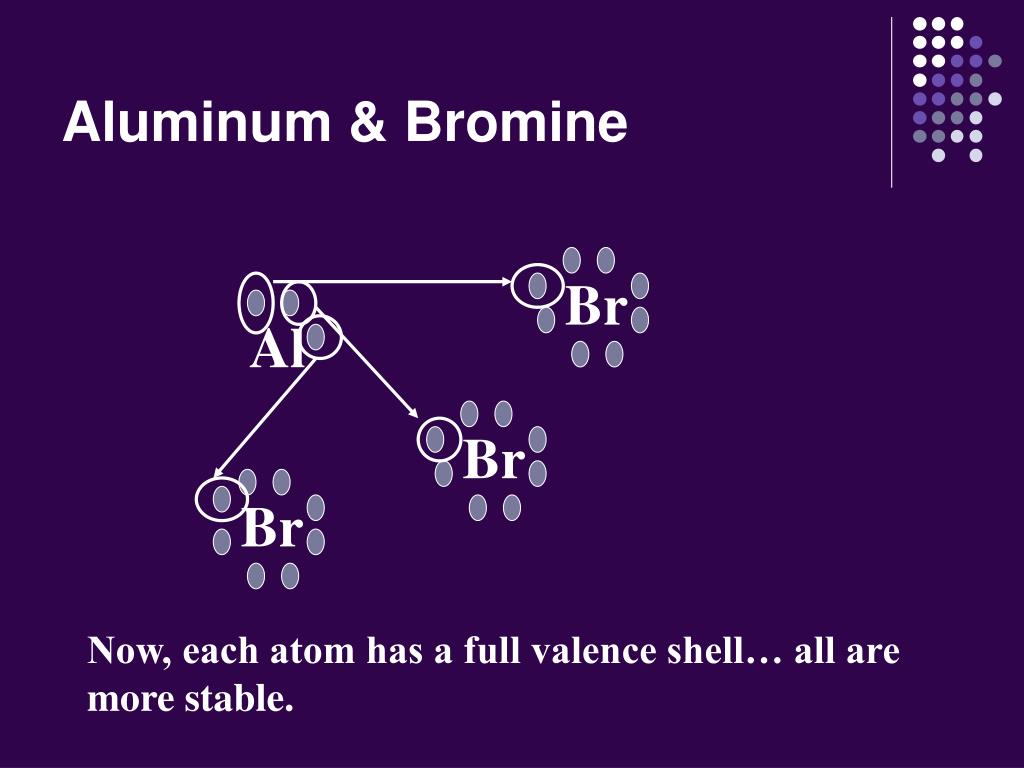
How Many Bonds Does Bromine Form - Web how many covalent bonds does a bromine atom form? Each atom contributes one electron to the bond, and share these bonding. Web typically, the atoms of group 4a form 4 covalent bonds; Web to do that, a bromine atom forms a covalent bond with another bromine atom. Get deals and low prices on bromine tablets 1 inch at amazon The valence electrons in al is three. Web posted sep 14, 2022. These four elements are widely used when it comes to drawing lewis structures at. Web bromine is a diatomic molecule and contains only bromine atoms. Bond energies to bromine tend to be lower than those to chlorine but higher than those to iodine, and bromine is a weaker oxidising agent than chlorine but a stronger one than iodine. Bromine compounds are compounds containing the element bromine (br). And group 7a form one bond. Web posted sep 14, 2022. It can form 1, 2, or 3 bonds with other atoms. Web typically, the atoms of group 4a form 4 covalent bonds; Group 6a form 2 bonds; Bromine atoms have a strong tendency to gain. Web posted sep 14, 2022. 109 degrees which of the following is the. Web to do that, a bromine atom forms a covalent bond with another bromine atom. Group 6a form 2 bonds; Web the atomic number of al is 13, and its electronic configuration is 1s22p22p63s23p1. Bromine compounds are compounds containing the element bromine (br). The maximum number of bonds. Bond energies to bromine tend to be lower than those to chlorine but higher than those to iodine, and bromine is a weaker oxidising agent than chlorine. Bromine is a halogen and has 7 valence electrons. Web remember that the dash, also referred to as a single bond, represents a pair of electrons. This can be seen from the standard electrode potentials of the x2/x couples (f, +2.866 v; Get deals and low prices on bromine tablets 1 inch at amazon A single bond and two double. Web bromine will normally form one covalent bond. Chemical element in the periodic table of elements. And group 7a form one bond. It can form 1, 2, or 3 bonds with other atoms. 109 degrees which of the following is the. Ad enjoy great deals and discounts on an array of products from various brands. Bromine atoms have a strong tendency to gain. Chlorine (cl 2 ) was the first halogen to be discovered in 1774,. Bond energies to bromine tend to be lower than those to chlorine but higher than those to iodine, and bromine is a weaker oxidising agent. Web bromine is a 35. Web let's illustrate how a covalent bond forms between iodine and bromine, with the understanding that each atom only needs one more electron to complete an octet. Web how many bonds does bromine form? These four elements are widely used when it comes to drawing lewis structures at. Group 6a form 2 bonds; Bromine, which belongs to group 17 and period four of the periodic table, has seven outer shell or valence Group 5a form 3 bonds; Group 6a form 2 bonds; It has 35 protons and 35 electrons in the atomic structure. Web how many covalent bonds does a bromine atom form? 109 degrees which of the following is the. Web bromine will normally form one covalent bond. Web if either iodine or bromine were to given up valence electrons to form a cation, they would have to give up all seven valence electrons to reveal the next Bromine atoms have a strong tendency to gain. Web bromine is a diatomic molecule. Web bromine exists as a diatomic molecule with the chemical formula br 2 that belongs to the halogen group. Ad enjoy great deals and discounts on an array of products from various brands. Bromine, which belongs to group 17 and period four of the periodic table, has seven outer shell or valence Bromine is intermediate in reactivity between chlorine and. Which of the following situations meet the bonding requirement for carbon atoms. Web bromine exists as a diatomic molecule with the chemical formula br 2 that belongs to the halogen group. The maximum number of bonds. Web bromine is a diatomic molecule and contains only bromine atoms. The valence electrons in al is three. It is the process of two or more atoms coming together. 109 degrees which of the following is the. This can be seen from the standard electrode potentials of the x2/x couples (f, +2.866 v; Bromine compounds are compounds containing the element bromine (br). Each atom contributes one electron to the bond, and share these bonding. Bromine, which belongs to group 17 and period four of the periodic table, has seven outer shell or valence. It can form 1, 2, or 3 bonds with other atoms. Bromine, which belongs to group 17 and period four of the periodic table, has seven outer shell or valence These four elements are widely used when it comes to drawing lewis structures at. Web remember that the dash, also referred to as a single bond, represents a pair of electrons. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos and images. Bromine is intermediate in reactivity between chlorine and iodine, and is one of the most reactive elements. A single bond and two double bonds. Web the atomic number of al is 13, and its electronic configuration is 1s22p22p63s23p1. Group 6a form 2 bonds;How Can We Find A Electron Configuration For Bromine (Br)
Bromine Valence Electrons Bromine Valency (Br) Dot Diagram
PPT Chemistry Unit 2 PowerPoint Presentation, free download ID3668529
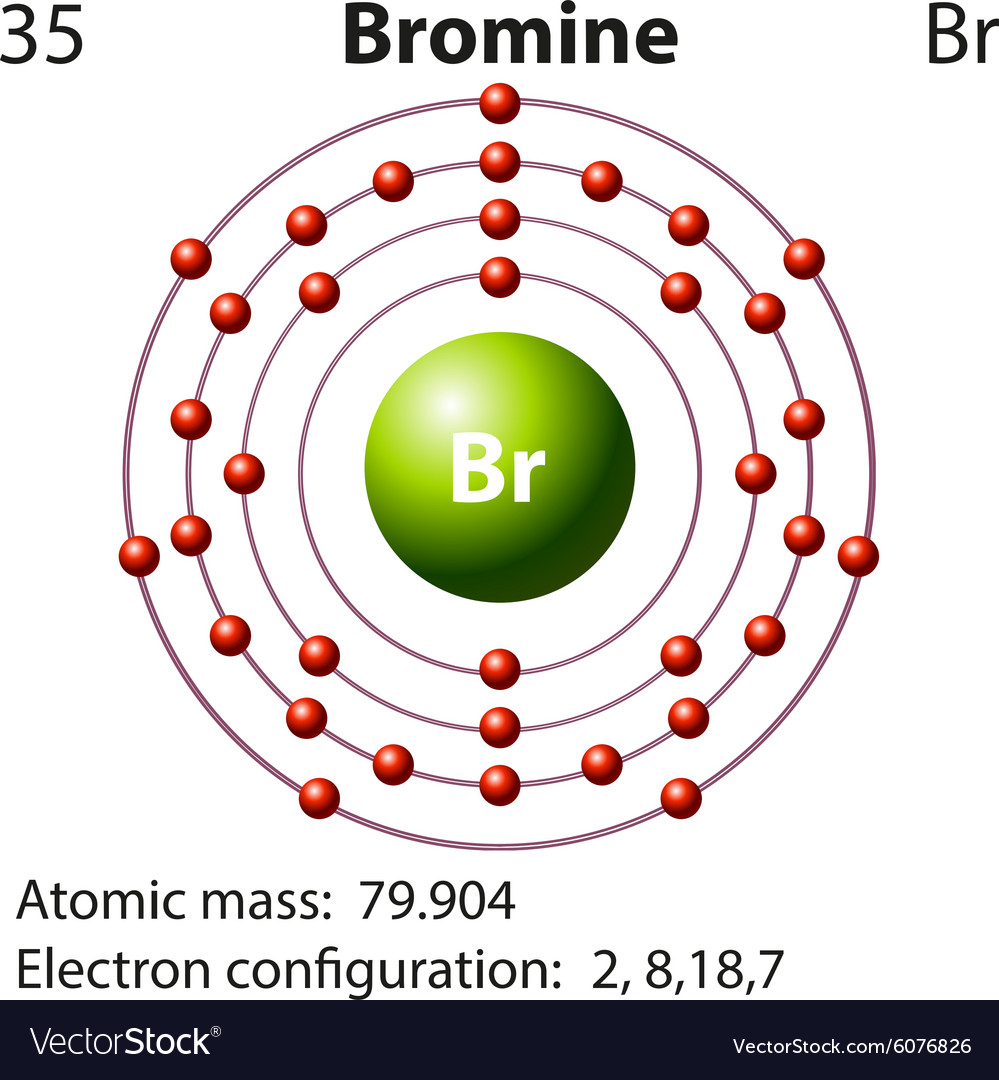
Symbol and electron diagram for bromine Royalty Free Vector
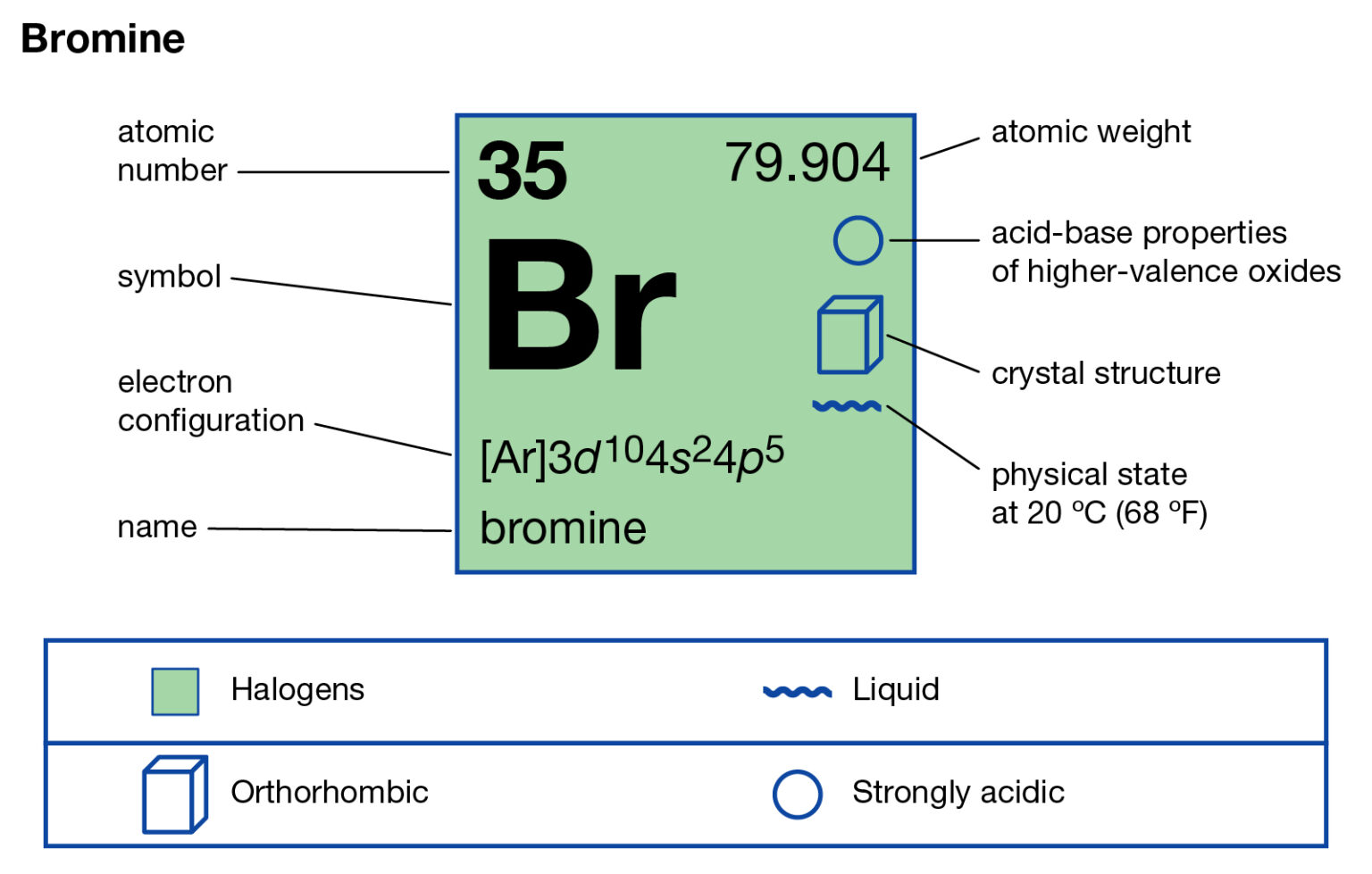
Bromine Periodic Table and Atomic Properties
Bromine Electron Configuration (Br) with Orbital Diagram
How Many Valence Electrons Does Bromine (Br) Have? [Valency of Bromine]
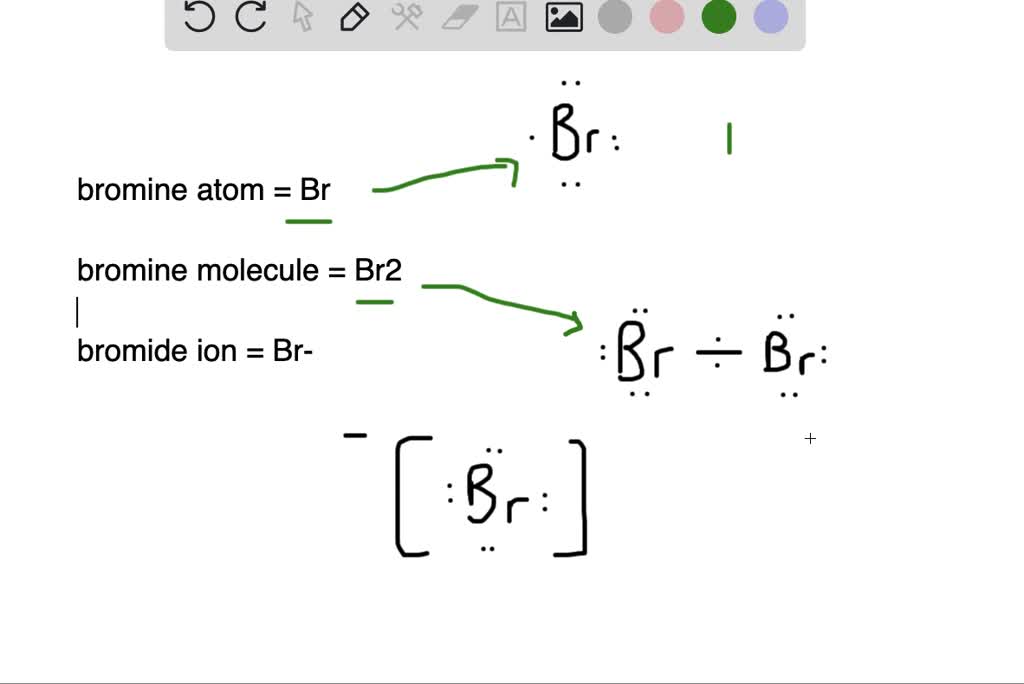
SOLVEDWhat is the difference between (a) a bromine atom, (b) a bromine
How Can We Find A Electron Configuration For Bromine (Br)
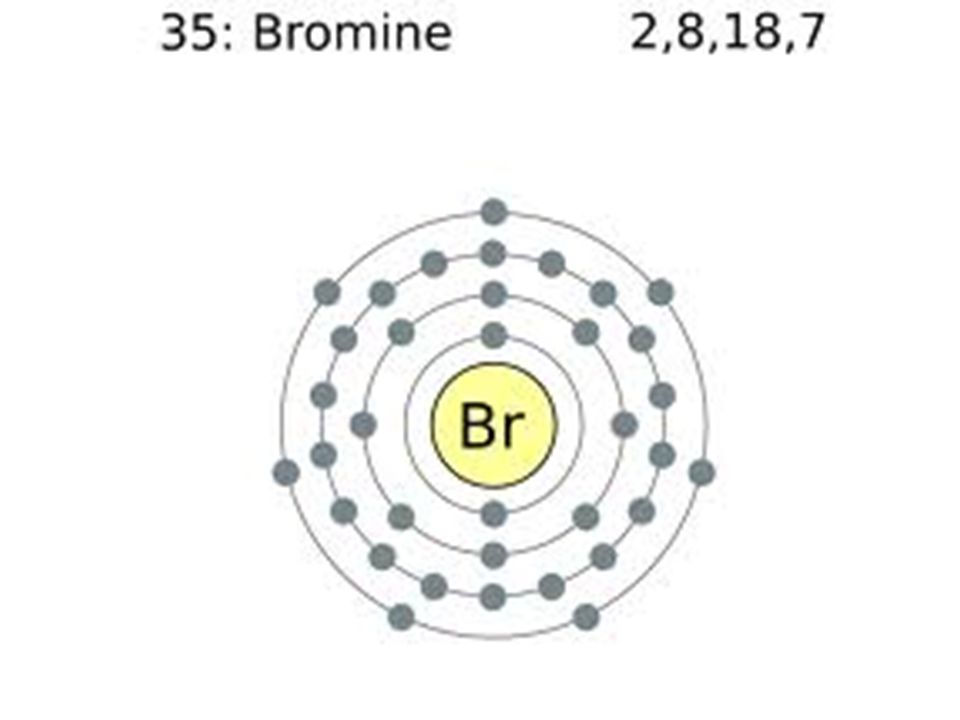
Symbol and electron diagram for Bromine illustration Stock Vector Image
Related Post:


![How Many Valence Electrons Does Bromine (Br) Have? [Valency of Bromine]](https://1.bp.blogspot.com/-ynLTJPSFkNY/YBXNXDx8ACI/AAAAAAAADZU/h1jbYW5duhMr7iiZUWhxGFBBUoDEUho1wCLcBGAsYHQ/w1600/Screenshot%2B%252878%2529-min.png)