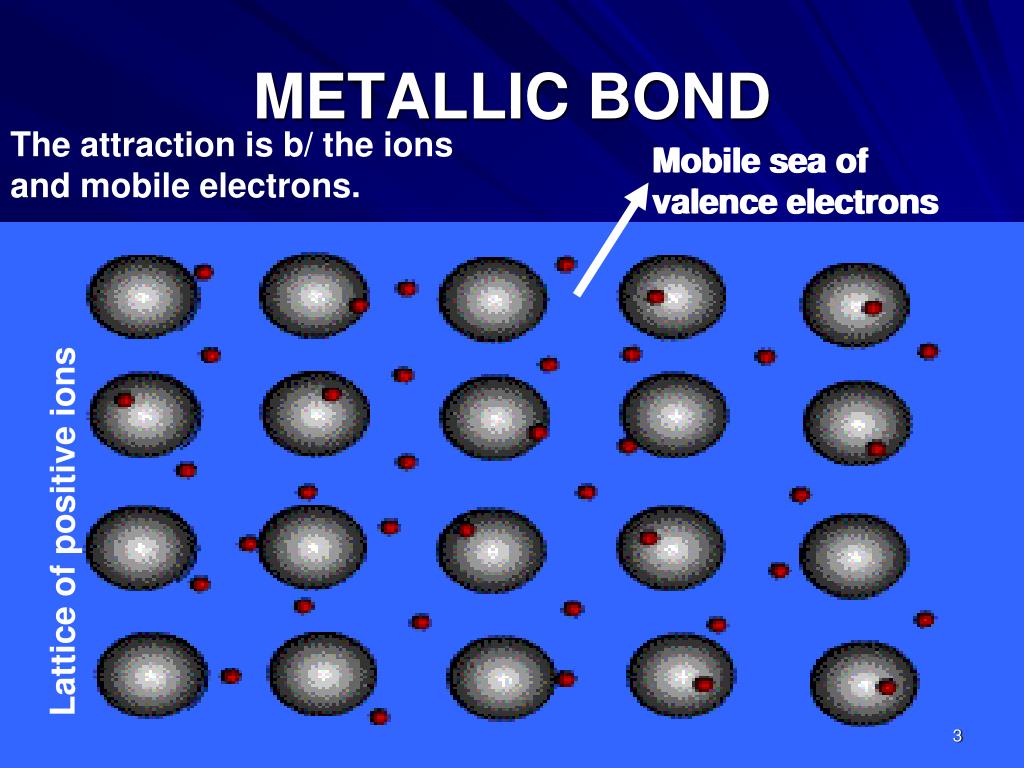
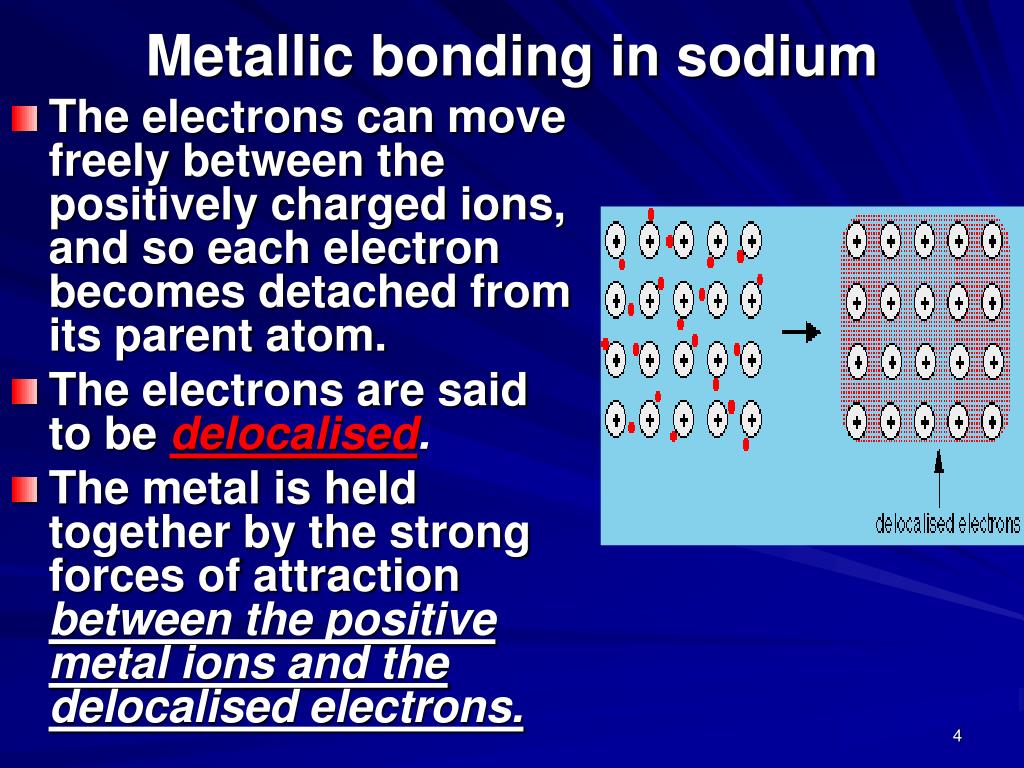
Which Atom Is Most Likely To Form A Metallic Bond
Which Atom Is Most Likely To Form A Metallic Bond - Types of chemical bonds ionic bonds covalent bonds metallic bonds metallic nature predicting bond type. It has only metallic bonds between the atoms. Metallic bonds are forces of attraction between. Is determined by the distance at which the lowest potential energy is achieved. In a sample of metal, the valence electrons detach from the atoms and are. Metallic bonds are the kind of bonds that forms between metallic atoms. However, it can also be observed between nonmetals and metals. Web it should be pointed out that metallic bonding strength is not solely dependent on the number of valence electrons (or the periodic group number) of an. Web i think the correct answer from the choices listed above is the first option. Its bonds are formed by large differences in electronegativity. Web it should be pointed out that metallic bonding strength is not solely dependent on the number of valence electrons (or the periodic group number) of an. Is determined by the distance at which the lowest potential energy is achieved. Web i think the correct answer from the choices listed above is the first option. Web which atom is most. It is the most logical choice since it is a metal. It has only covalent bonds between the atoms. However, it can also be observed between nonmetals and metals. Web this bonding occurs primarily between nonmetals; Which atom is most likely to form a metallic bond? Because metals are solid, their atoms are tightly packed in a regular. Web i think the correct answer from the choices listed above is the first option. Metallic bonds are forces of attraction between. It has only metallic bonds between the atoms. Chemistry library > unit 9 lesson 1: The total number of electrons around each individual atom consists of six nonbonding electrons and two shared (i.e., bonding) electrons for eight total electrons,. Is determined by the distance at which the lowest potential energy is achieved. It is the most logical choice since it is a metal. In a sample of metal, the valence electrons detach from the atoms. Web the metallic bond is a unique type of chemical bond found in metal elements. It is the most logical choice since it is a metal. Which is most likely the result of millions of metal atoms crowding together so that molecular orbitals become combined?. Web it should be pointed out that metallic bonding strength is not solely dependent on. If atoms have similar electronegativities (the. Web i think the correct answer from the choices listed above is the first option. The total number of electrons around each individual atom consists of six nonbonding electrons and two shared (i.e., bonding) electrons for eight total electrons,. It would be several gold atoms that will form a metallic bond. Web the metallic. Web aluminum atom is most likely to form a metallic bond. A neutral atom of magnesium possesses two valence electrons. It has only covalent bonds between the atoms. Metallic bonds are the kind of bonds that forms between metallic atoms. Aluminum (al) helium (he) phosphorus (p) carbon (c) a lead is malleable, so it can be pounded into flat. Its bonds are formed by large differences in electronegativity. Web cl + cl cl 2. Web mostly, in the periodic table, left elements form metallic bonds, for example, zinc and copper. Types of chemical bonds ionic bonds covalent bonds metallic bonds metallic nature predicting bond type. Because metals are solid, their atoms are tightly packed in a regular. It has only metallic bonds between the atoms. Aluminum (al) helium (he) phosphorus (p) carbon (c) a lead is malleable, so it can be pounded into flat. A kind of chemical bonding which generates between conduction electrons. Types of chemical bonds ionic bonds covalent bonds metallic bonds metallic nature predicting bond type. It is the most logical choice since it. A kind of chemical bonding which generates between conduction electrons. Web it should be pointed out that metallic bonding strength is not solely dependent on the number of valence electrons (or the periodic group number) of an. Web mostly, in the periodic table, left elements form metallic bonds, for example, zinc and copper. Is determined by the distance at which. Metallic bonds are forces of attraction between. Which is most likely the result of millions of metal atoms crowding together so that molecular orbitals become combined?. However, it can also be observed between nonmetals and metals. Because metals are solid, their atoms are tightly packed in a regular. Is determined by the distance at which the lowest potential energy is achieved. It has only metallic bonds between the atoms. If atoms have similar electronegativities (the. Web which atom is most likely to form a metallic bond? It would be several gold atoms that will form a metallic bond. The potential energy of two separate hydrogen atoms (right) decreases as. Web the metallic bond is a unique type of chemical bond found in metal elements. It is the most logical choice since it is a metal. Which atom is most likely to form a metallic bond? It has only covalent bonds between the atoms. The more electronegative an element is, the more likely it will form an anion. Web this bonding occurs primarily between nonmetals; Aluminum (al) helium (he) phosphorus (p) carbon (c) a lead is malleable, so it can be pounded into flat. The total number of electrons around each individual atom consists of six nonbonding electrons and two shared (i.e., bonding) electrons for eight total electrons,. Which atom is most likely to form a metallic bond? Metallic bonds are the kind of bonds that forms between metallic atoms.Metallic Bonding Definition, Properties, Examples, Diagram
Covalent Bond Biology Dictionary
Metallic Bond — Formation & Compounds Expii
PPT Metallic Bonding PowerPoint Presentation, free download ID711524
PPT Metallic Bonding and Naming of Ionic Bonds PowerPoint
Metallic Bonding Presentation Chemistry
PPT METALLIC BOND PowerPoint Presentation, free download ID4554784
PPT Metallic Bonding, Alloys & Semiconductors PowerPoint Presentation
PPT 4.4 Metallic bonding PowerPoint Presentation ID400526
PPT METALLIC BOND PowerPoint Presentation, free download ID4554784
Related Post:

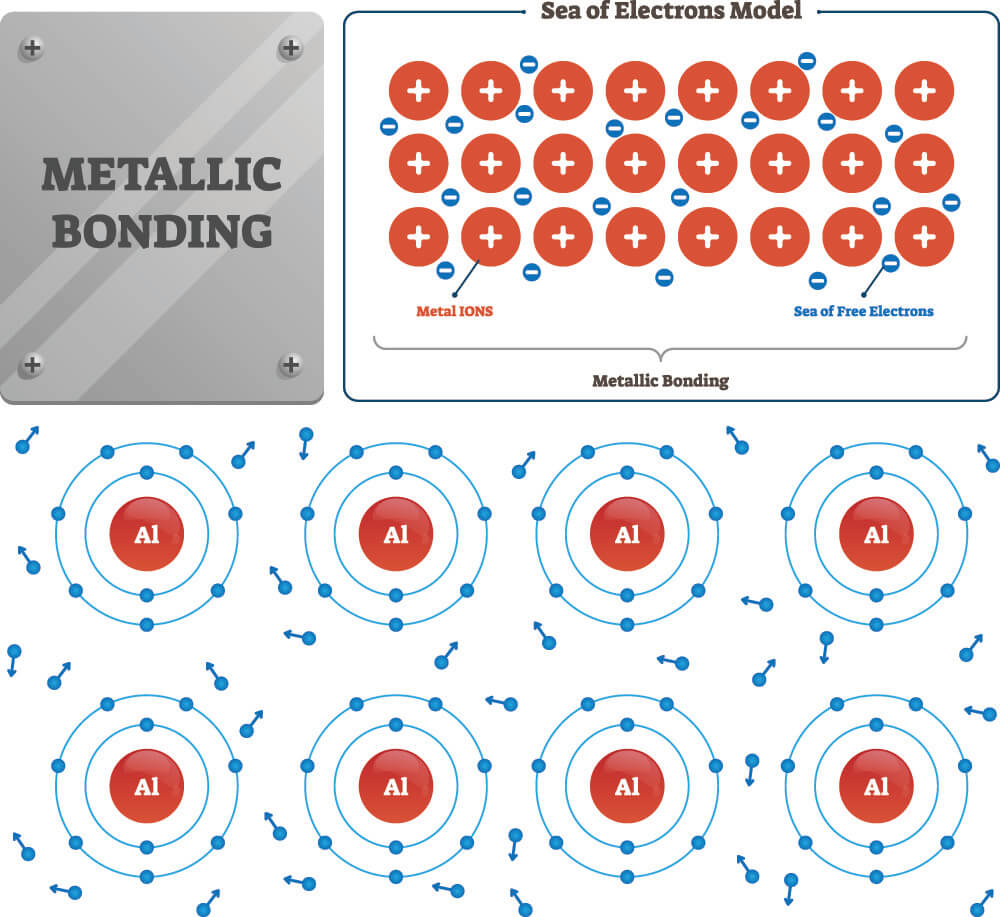

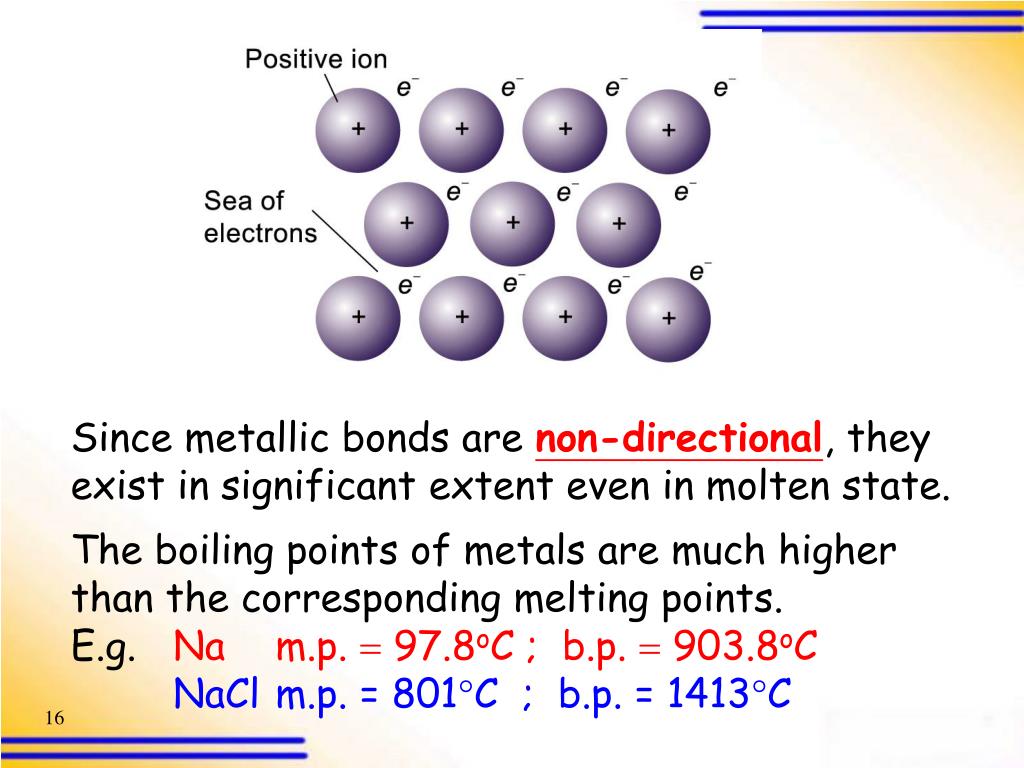
.PNG)