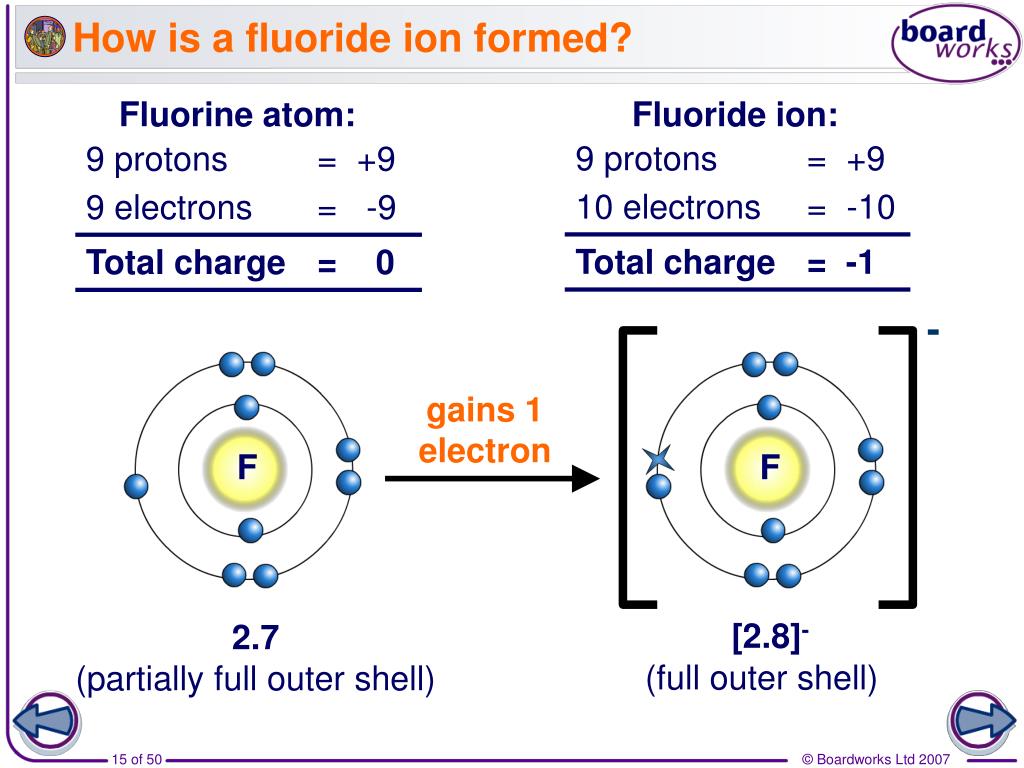
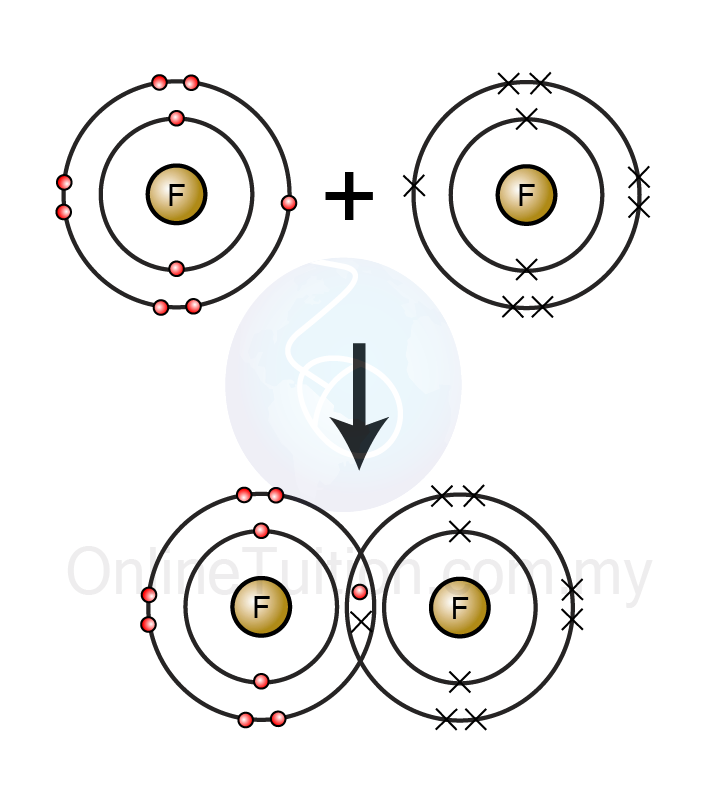
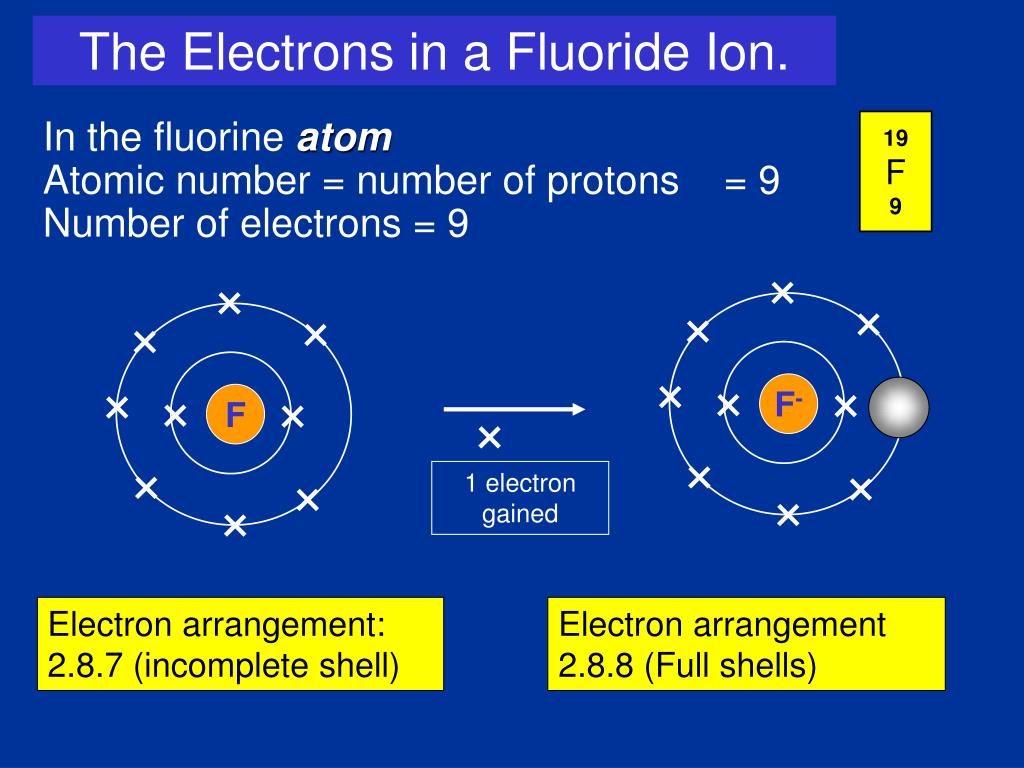
How Many Bonds Can Fluorine Form
How Many Bonds Can Fluorine Form - This problem has been solved! The number of electrons required to obtain an. Web how many bonds can fluorine form? Web how many single covalent bonds can fluorine form? You'll get a detailed solution from a subject matter expert that helps. The atom would be energetically stable if it achieved an 8 electron count. Fluorine's bonds are technically covalent, but it is such a strongly electron withdrawing group that for all. Web as a stable electron configuration requires 8 electrons total, fluorine must form 1 bond i.e. Web oxygen and other atoms in group 6a (16) obtain an octet by forming two covalent bonds. Web oxygen and other atoms in group 6a (16) obtain an octet by forming two covalent bonds. Web a covalent bond is the force of attraction that holds together two nonmetal atoms that share a pair of electrons. On the left is a fluorine atom with seven. You'll get a detailed solution from a subject matter expert that helps. There are six fluorine atoms that form bonds. It is also able to form hydrogen. Web fluorine can form covalent bonds with other nonmetal elements and can also form ionic bonds with metals to form fluorides. Web does fluorine ever form a double or triple bond? It is also able to form hydrogen. Web oxygen and other atoms in group 6a (16) obtain an octet by forming two covalent bonds. Two core and seven valence. Web how many single covalent bonds can fluorine form? Web how many bonds can fluorine form? A) 1 b) 2 c) 3 d) 4 a which of the following ionic compounds has the largest lattice energy (i.e., the lattice energy most. When carbon reacts with fluorine, the reaction is complex and. Ask question asked 5 years, 10 months ago modified. Web a covalent bond is the force of attraction that holds together two nonmetal atoms that share a pair of electrons. Web fluorine forms covalent bonds with carbon, which sometimes form into stable aromatic rings. Web how many bonds can fluorine form? The polar nature of the bond means that there is a large. Fluorine and the other halogens in. Web fluorine forms covalent bonds with carbon, which sometimes form into stable aromatic rings. Fluorine's bonds are technically covalent, but it is such a strongly electron withdrawing group that for all. Fluorine and the other halogens in group 7a (17) have seven valence. On the left is a fluorine atom with seven. Web how many covalent bonds can an atom. Fluorine's bonds are technically covalent, but it is such a strongly electron withdrawing group that for all. Web does fluorine ever form a double or triple bond? When carbon reacts with fluorine, the reaction is complex and. How many covalent bonds are predicted for fluorine? Web writing lewis structures and such things, fluorine always forms single bonds. Web abstract a central dogma is that fluorine is the most electro negative element in the pse and would thusact as a pure electron acceptor. There are six fluorine atoms that form bonds. Web the high electronegativity of fluorine means that it forms a single electron pair bond polar bond with a high ionic character. In many respects, metal fluorides. Owing to its high electronegativity, fluorine stabilizes metals in higher oxidation states with high m:halide ratios. The number of electrons required to obtain an. On the left is a fluorine atom with seven. Web a covalent bond is the force of attraction that holds together two nonmetal atoms that share a pair of electrons. Bonding molecules, such like breathable oxygen. Sources, facts, uses, scarcity (sri),. It is also able to form hydrogen. When carbon reacts with fluorine, the reaction is complex and. Ask question asked 5 years, 10 months ago modified 4 months ago viewed 8k times 4 does fluorine ever. Fluorine's bonds are technically covalent, but it is such a strongly electron withdrawing group that for all. Two core and seven valence. Web oxygen and other atoms in group 6a (16) obtain an octet by forming two covalent bonds. The atom would be energetically stable if it achieved an 8 electron count. Atomic fluorine has 7 valence electrons; In many respects, metal fluorides are more similar to oxides, often having similar bonding and crystal structures. This problem has been solved! Web the high electronegativity of fluorine means that it forms a single electron pair bond polar bond with a high ionic character. Web how many single covalent bonds can fluorine form? Web oxygen and other atoms in group 6a (16) obtain an octet by forming two covalent bonds. You'll get a detailed solution from a subject matter expert that helps. Atomic fluorine has 7 valence electrons; When carbon reacts with fluorine, the reaction is complex and. Web fluorine and the other halogens in group 7a have seven valence electrons and can obtain an octet by forming one covalent bond. Fluorine and the other halogens in group 7a (17) have seven valence. Two core and seven valence. On the left is a fluorine atom with seven. How many covalent bonds are predicted for fluorine? Web how many covalent bonds can an atom of fluorine form? There are six fluorine atoms that form bonds. Web how many bonds can fluorine form? Sources, facts, uses, scarcity (sri),. Web yes does fluorine usually form ionic or covalent bonds? Fluorine and the other halogens in group 7a (17) have seven valence electrons and can. Web a single covalent bond. Fluorine's bonds are technically covalent, but it is such a strongly electron withdrawing group that for all.Bonding Bonding and Chemical Interactions Training MCAT General
Covalent bonds Learning Lab
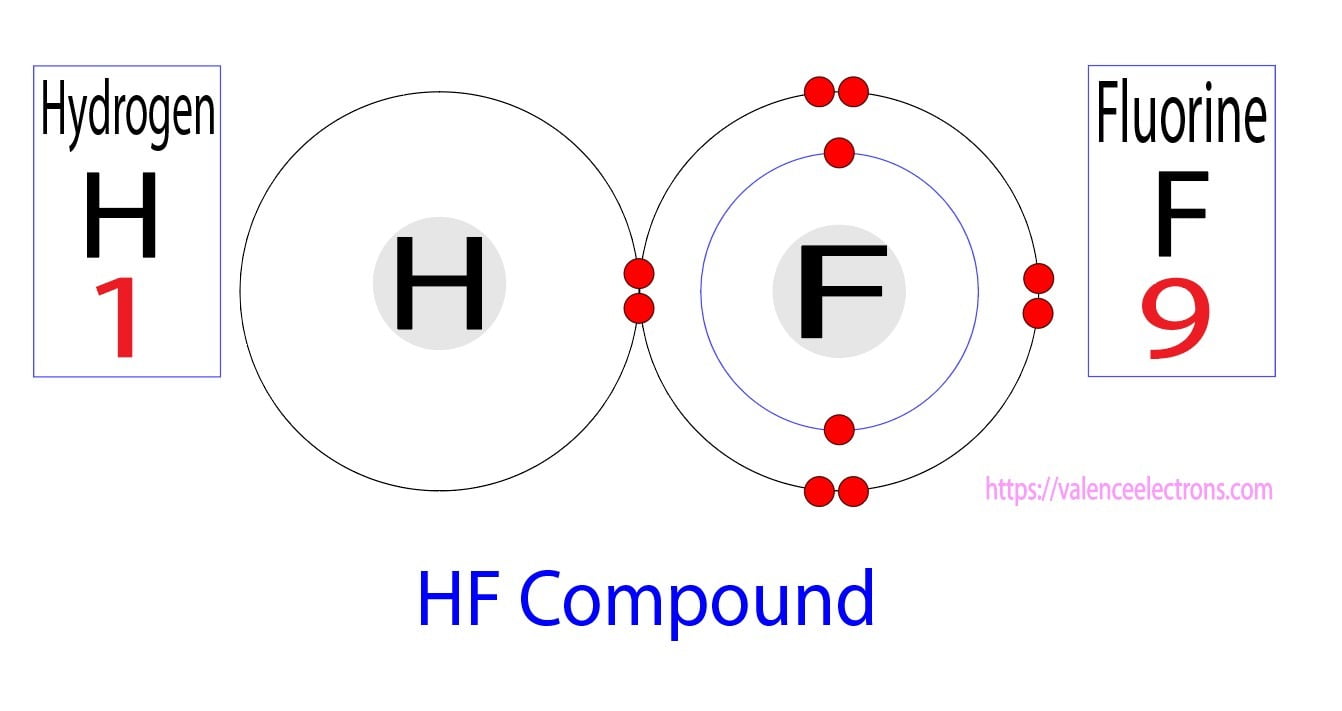
Hydrogen Bonding in Hydrogen fluoride (HF) YouTube
PPT Chemical Bonding and Nomenclature PowerPoint Presentation, free
PPT Ionic Bonding L.O. PowerPoint Presentation, free download ID
How to Find the Valence Electrons for Fluorine (F)?
Covalent Bonding (Molecules) Presentation Chemistry
Covalent Bonding (Biology) — Definition & Role Expii
PPT What are bonds? PowerPoint Presentation, free download ID5980343
Covalent Bonding SPM Chemistry
Related Post:
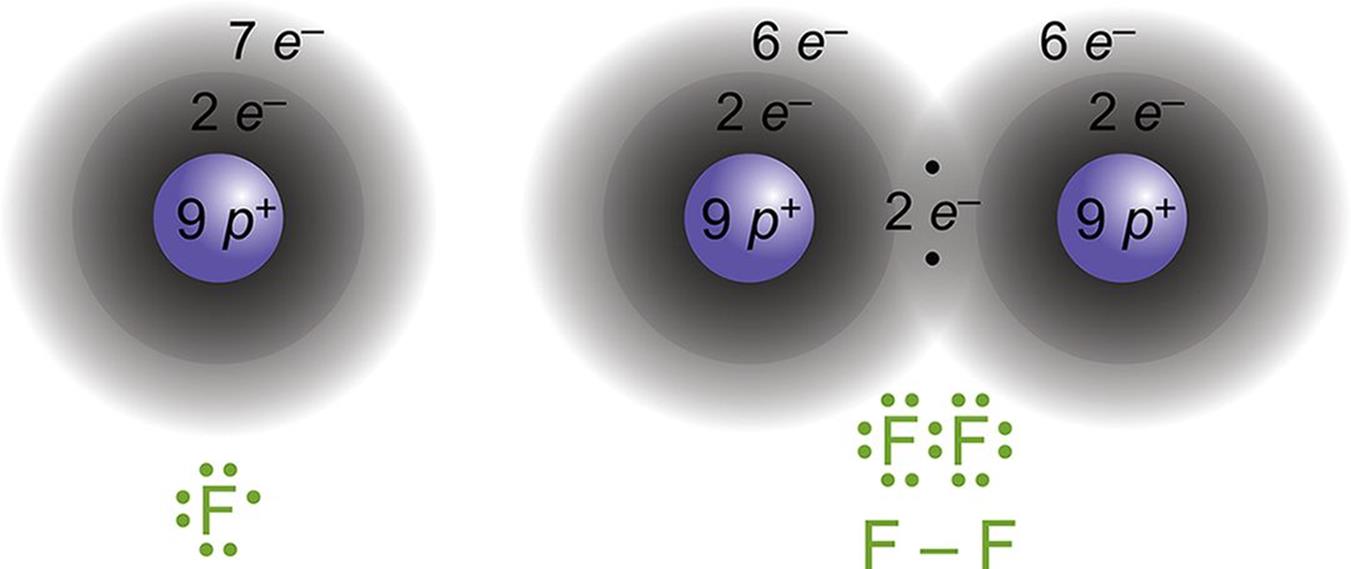


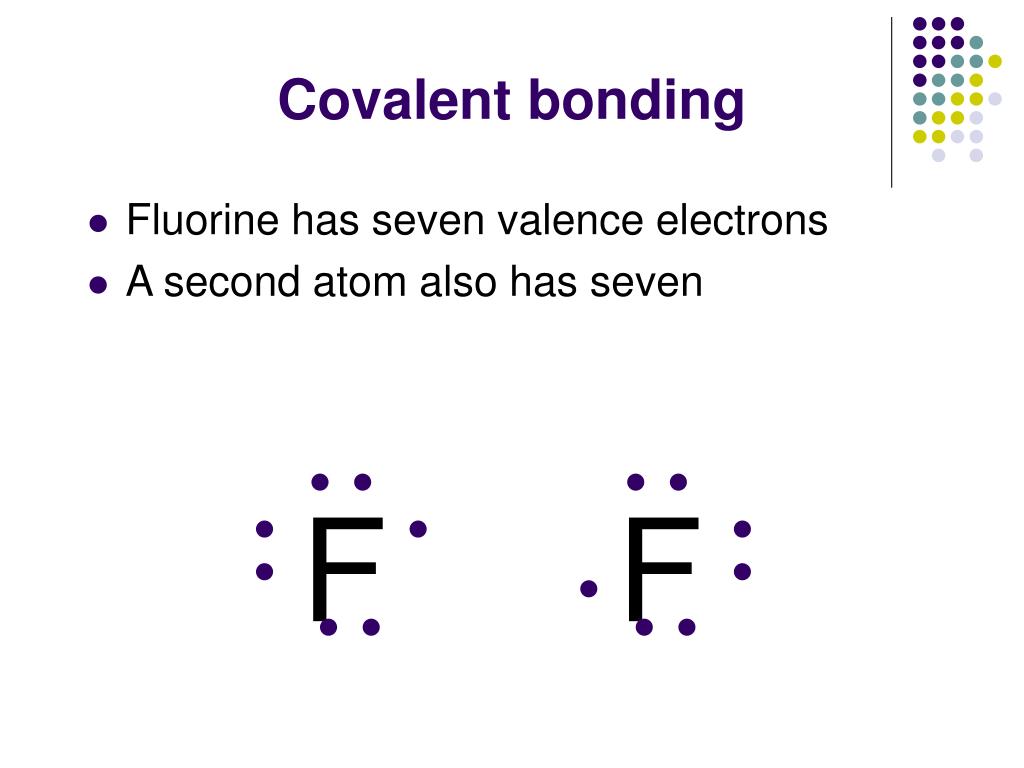
.PNG)