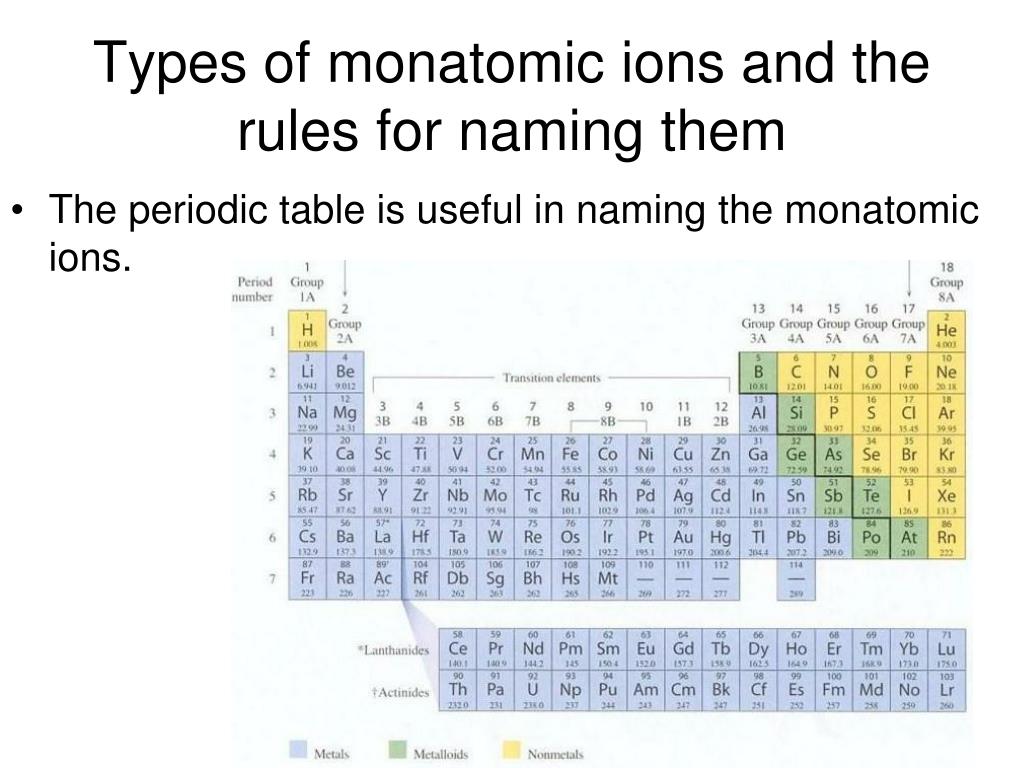
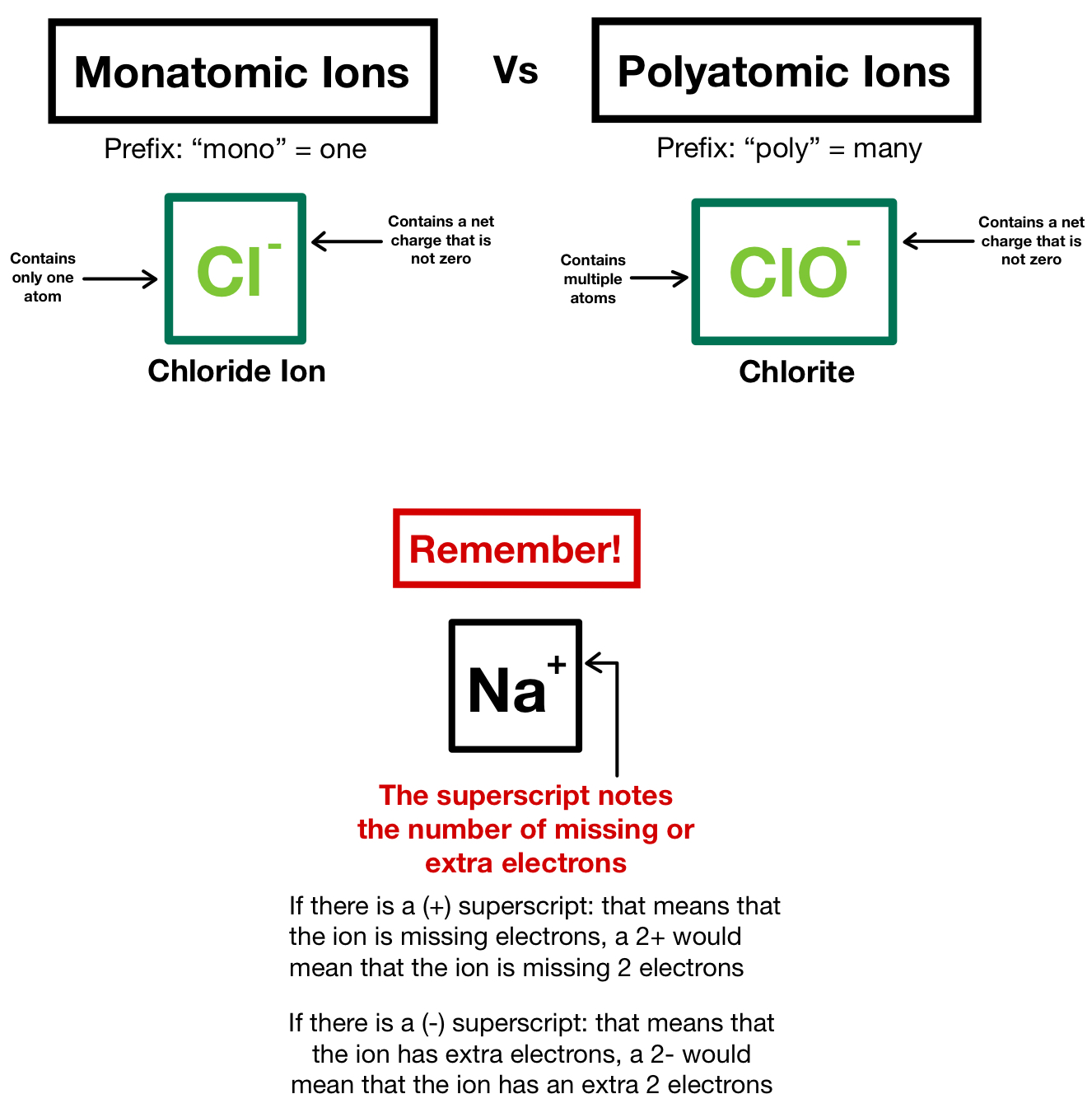
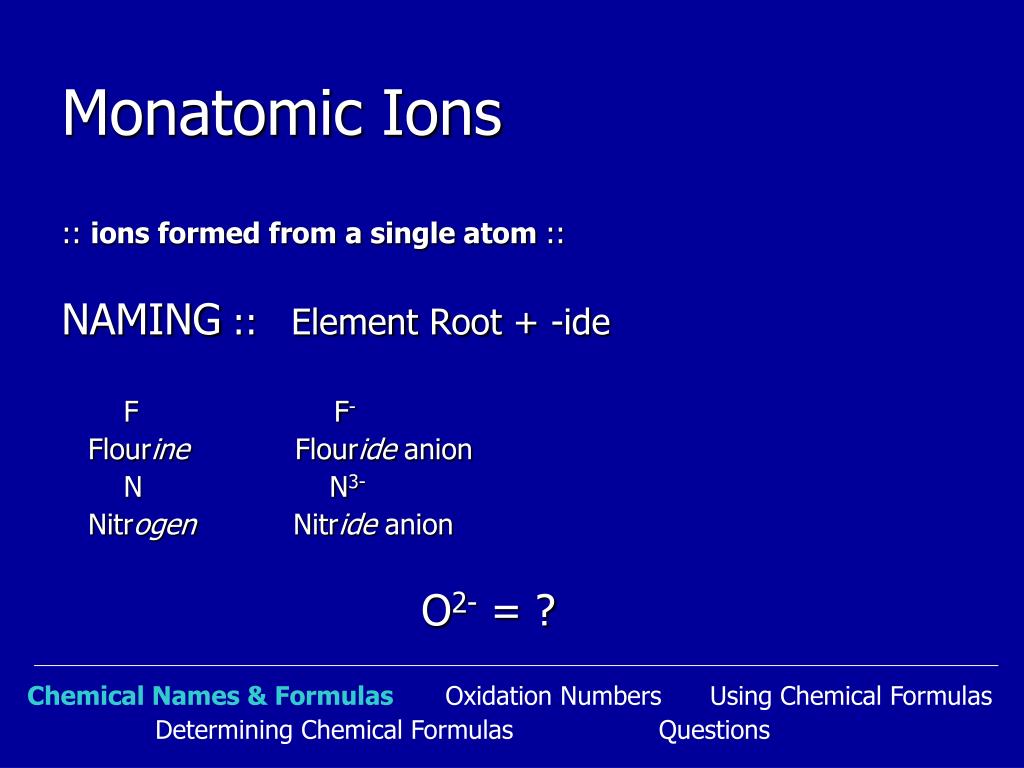
How Do Monatomic Ions Form
How Do Monatomic Ions Form - Web recognize characteristics of monatomic and polyatomic ions. Web a monatomic ion is an atom that has been ionized by gaining or losing electrons. Web moving from the far right to the left on the periodic table, elements often form anions with a negative charge equal to the number of groups moved left from the noble gases. The ion has a net charge because the total number of electrons is not balanced by the total. Web here are some tips on how to properly form monatomic ions: Names and formulas of ionic compounds. Web this lesson explains how to write monatomic ions. It explains how you can use the electronic configuration and the groups of elements to determine the types of ions that will be formed. Understand the periodic trends and electron configurations to predict the possible charges on monatomic. For example, fluorine will want 1 more electron to fill it's electron shell because it's one away from the closest noble gas. Web this lesson explains how to write monatomic ions. Group 1 elements form +1 ions group 2 elements form +2 ions group 13 elements form +3 ions group 14 elements form none group 15. Web the name of a monatomic cation is simply the name of the element followed by the word ion. The name of a monatomic cation is. We also find many polyatomic ions. Atoms are reactive because they have incomplete. Naming monatomic ions and ionic compounds. Thus, na + is the sodium ion, al 3 + is the aluminum ion, ca. Write the lewis symbols of the ions in each. Names and formulas of ionic compounds. Web a monatomic ion (also called simple ion [1] [2]) is an ion consisting of exactly one atom. Determine the number of subatomic. For example, fluorine will want 1 more electron to fill it's electron shell because it's one away from the closest noble gas. Web the natural formation of ions is: Naming monatomic ions and ionic compounds. Web here are some tips on how to properly form monatomic ions: It explains how you can use the electronic configuration and the groups of elements to determine the types of ions that will be formed. Names and formulas of ionic compounds. Web write the lewis symbols for the monatomic ions formed from the. Web how do monatomic ions form? Group 1 elements form +1 ions group 2 elements form +2 ions group 13 elements form +3 ions group 14 elements form none group 15. Thus, na + is the sodium ion, al 3 + is the aluminum ion, ca. Web a monatomic ion (also called simple ion [1] [2]) is an ion consisting. Web the ions that we have discussed so far are called monatomic ions, that is, they are ions formed from only one atom. The formation of a monatomic ion depends on its electron configuration. The ion has a net charge because the total number of electrons is not balanced by the total. Group 1 elements form +1 ions group 2. Understand the periodic trends and electron configurations to predict the possible charges on monatomic. The formation of a monatomic ion depends on its electron configuration. Web how do monatomic ions form? (a) cl (b) na (c) mg (d) ca (e) k (f) br (g) sr (h) f. Naming monatomic ions and ionic compounds. For example, fluorine will want 1 more electron to fill it's electron shell because it's one away from the closest noble gas. Web a monatomic ion is an atom that has been ionized by gaining or losing electrons. Web a monatomic ion (also called simple ion [1] [2]) is an ion consisting of exactly one atom. Compounds formed from positive. Explain how and why cations and anions are formed. It explains how you can use the electronic configuration and the groups of elements to determine the types of ions that will be formed. We also find many polyatomic ions. Write the lewis symbols of the ions in each. Web the natural formation of ions is: Web recognize characteristics of monatomic and polyatomic ions. Web a monatomic ion is an atom that has been ionized by gaining or losing electrons. For example, fluorine will want 1 more electron to fill it's electron shell because it's one away from the closest noble gas. A proton never moves from one atom to another. Web how do monatomic ions. The formation of a monatomic ion depends on its electron configuration. Thus, na + is the sodium ion, al 3 + is the aluminum ion, ca. Web moving from the far right to the left on the periodic table, elements often form anions with a negative charge equal to the number of groups moved left from the noble gases. Thus, na + is the sodium ion, al 3 + is the aluminum. Web a monatomic ion is an atom that has been ionized by gaining or losing electrons. Web the name of a monatomic cation is simply the name of the element followed by the word ion. Compounds formed from positive and. Group 1 elements form +1 ions group 2 elements form +2 ions group 13 elements form +3 ions group 14 elements form none group 15. Chemistry library > unit 1. Web the natural formation of ions is: Explain how and why cations and anions are formed. Web a monatomic ion (also called simple ion [1] [2]) is an ion consisting of exactly one atom. Web recognize characteristics of monatomic and polyatomic ions. For example, fluorine will want 1 more electron to fill it's electron shell because it's one away from the closest noble gas. Understand the periodic trends and electron configurations to predict the possible charges on monatomic. The ion has a net charge because the total number of electrons is not balanced by the total. Atoms are reactive because they have incomplete. Web here are some tips on how to properly form monatomic ions: Write the lewis symbols of the ions in each. Web how do monatomic ions form?IONS (monatomic) Learn the easy way how to form/write monatomic ions
PPT Monatomic Ions PowerPoint Presentation, free download ID3112818
Polyatomic Ions — Nomenclature & Compounds Expii
PPT Monatomic Ions PowerPoint Presentation, free download ID6687141
Monatomic Ion Easy Science Easy science, Ap chemistry, Chemistry
Give Two Examples of Monatomic Cation McCarron Theyess
PPT Chapter 9 Chemical Names and Formulas Section 9.1 Naming Ions
PPT Chemical Formulas and Chemical Compounds PowerPoint Presentation
PPT Chapter 7 Chemical Formulas and Names PowerPoint Presentation
PPT Chapter 7 Chemical Formulas and Chemical Compounds PowerPoint
Related Post: