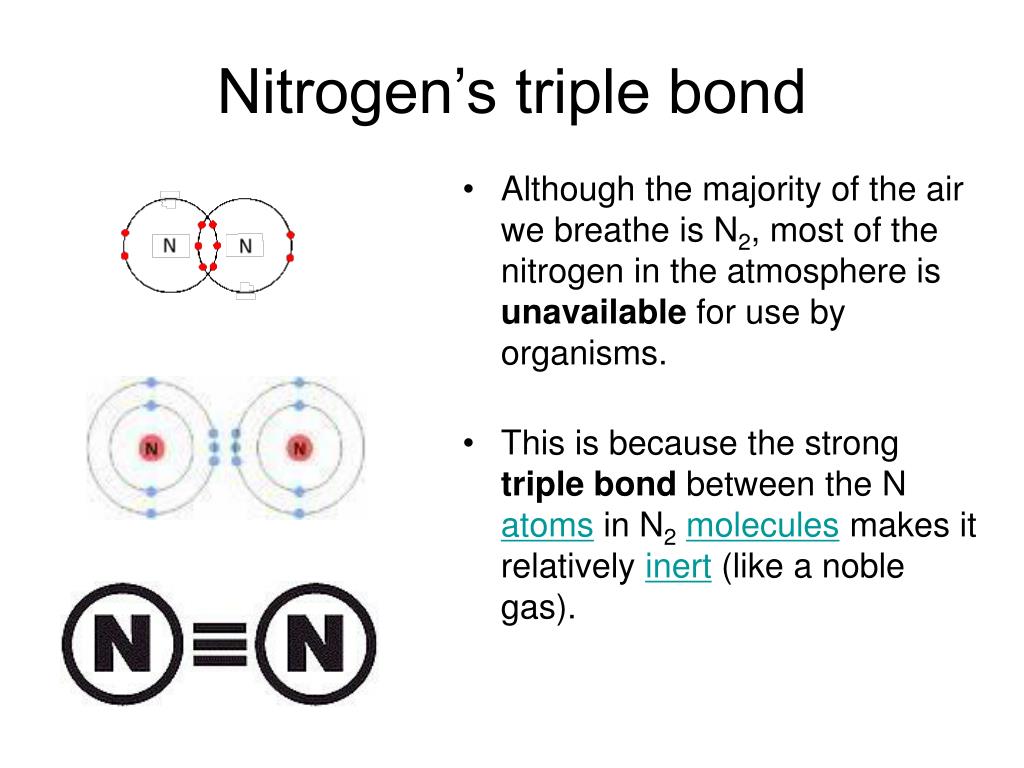
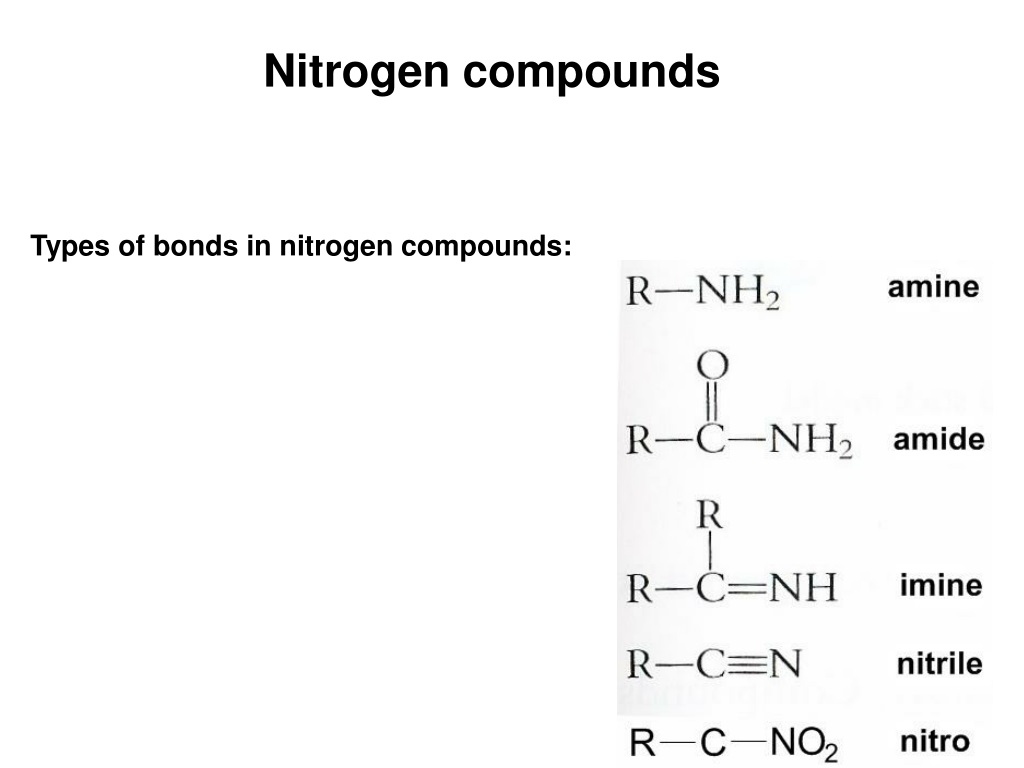
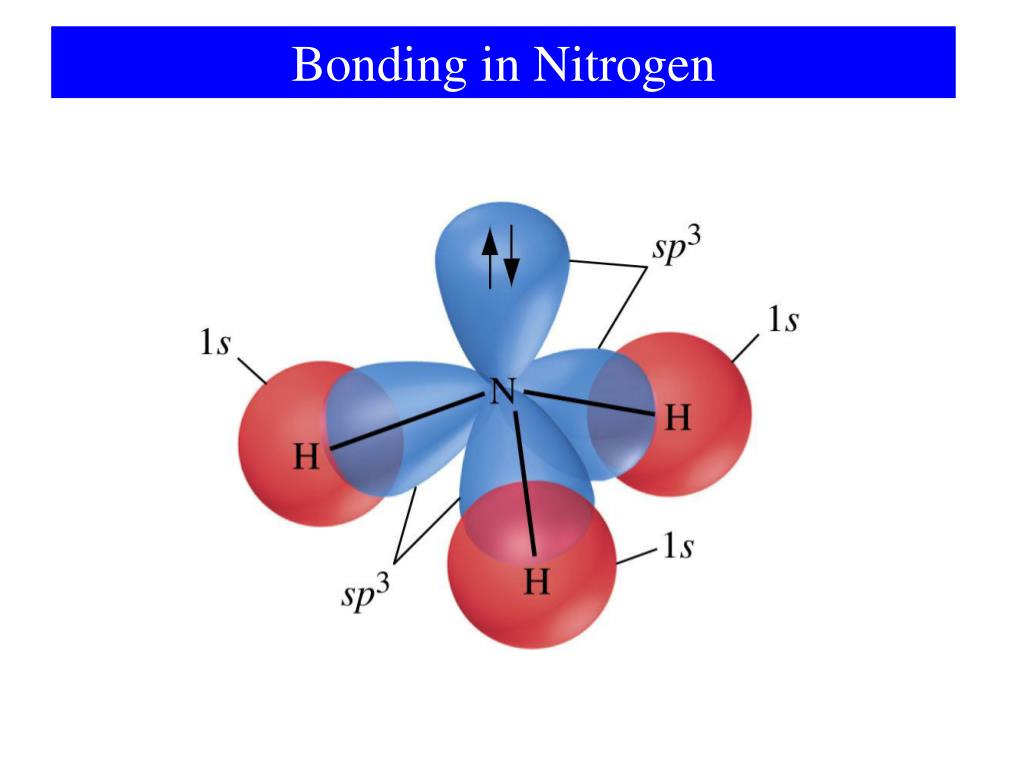
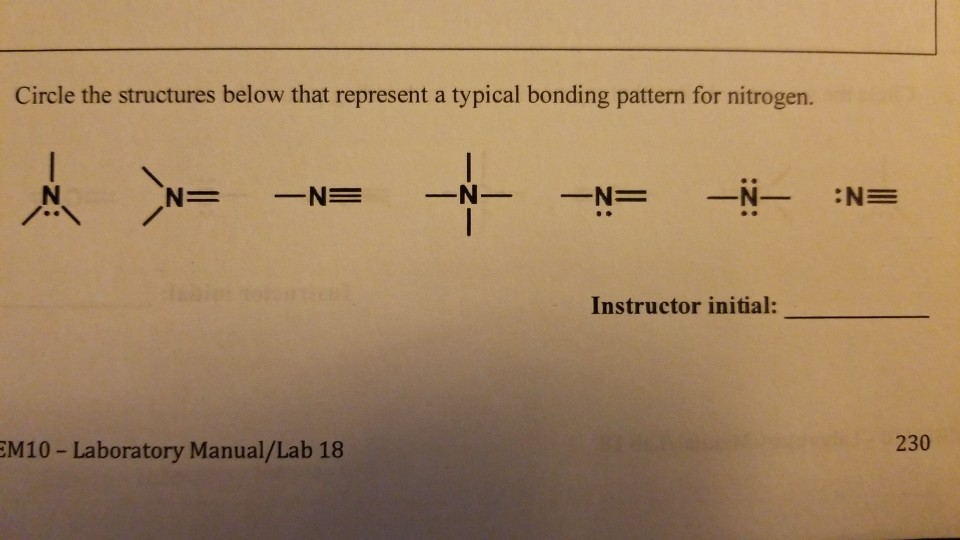
Can Nitrogen Form 4 Bonds
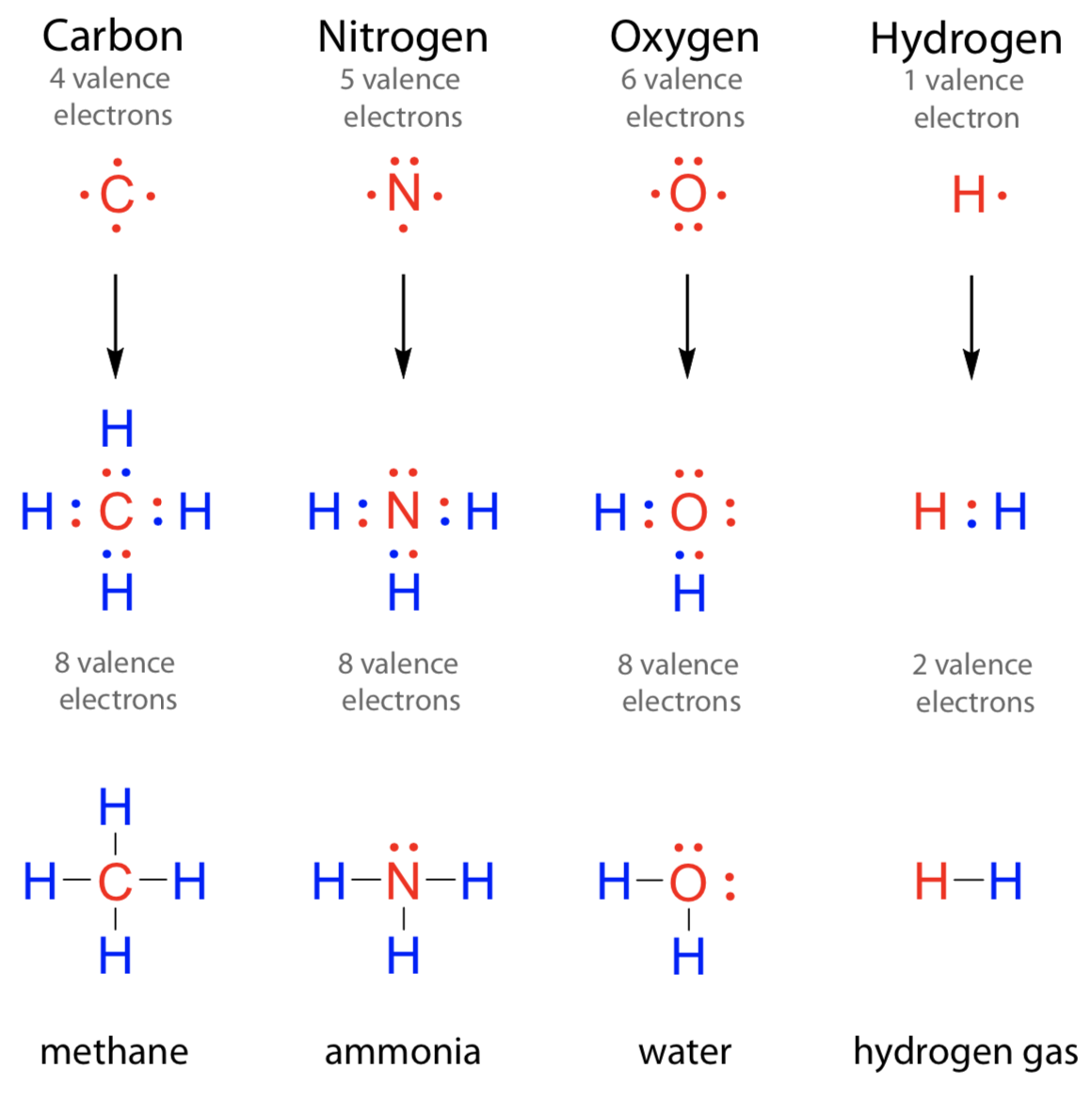
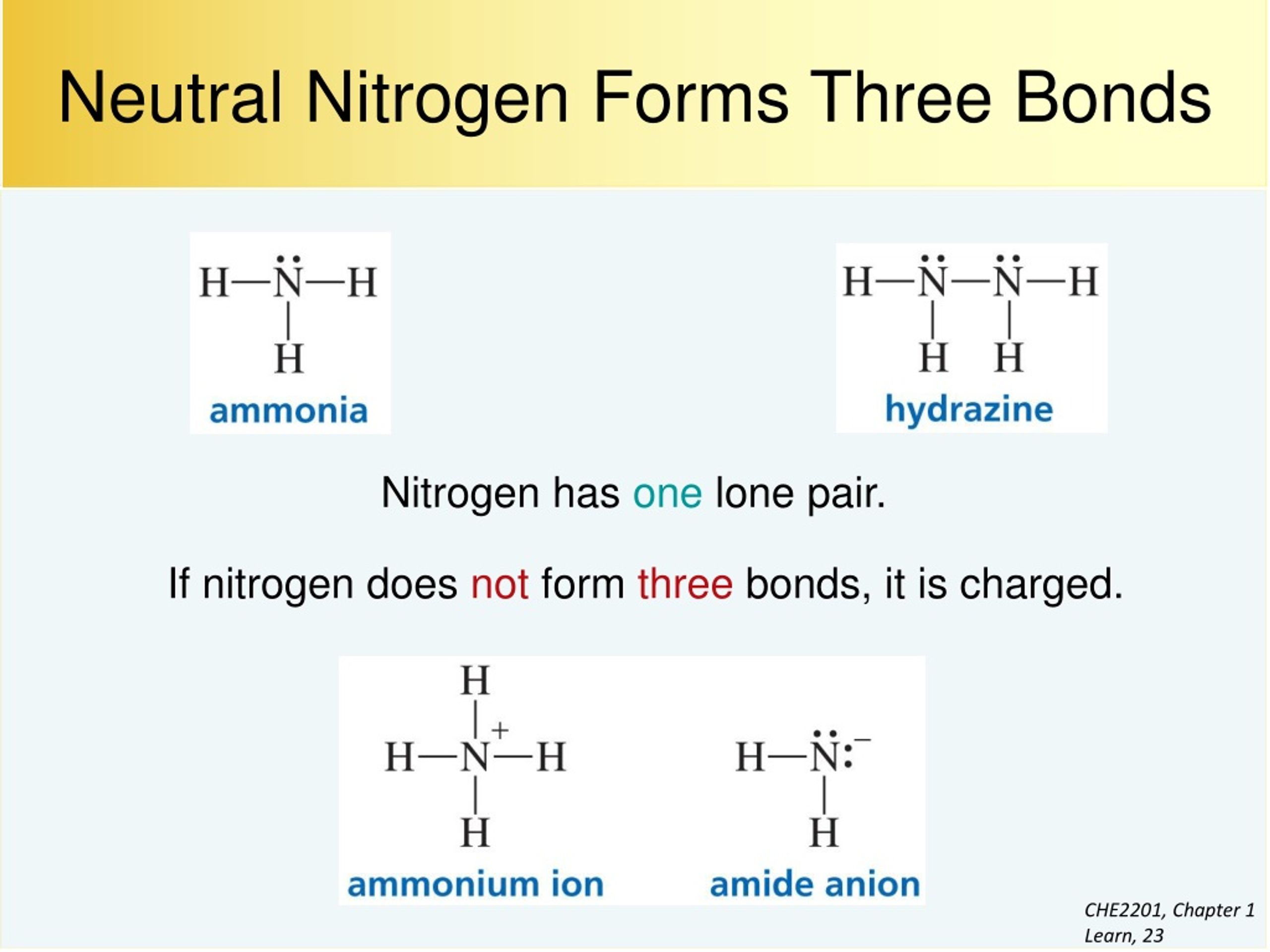
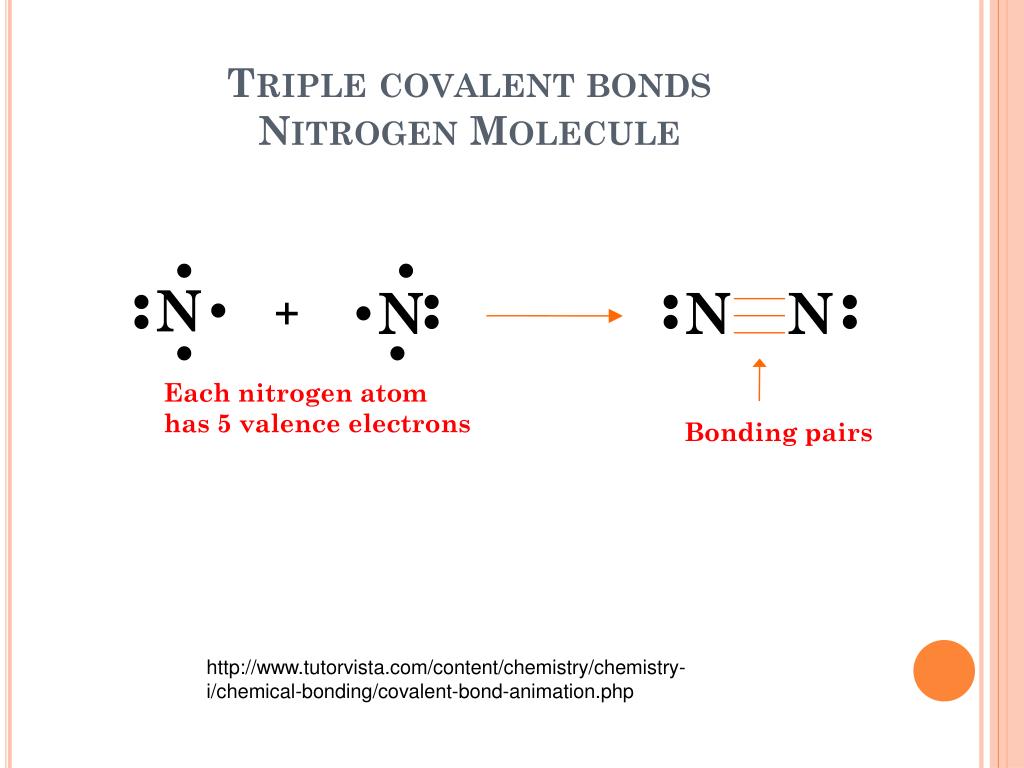
Can Nitrogen Form 4 Bonds - Web nitrogen (n 2) reacts with oxygen (o 2), the compound n o 2 can be formed as a product. For example, water, (\(\ce{h2o}\)), has two covalent bonds between a single oxygen atom and two. These are normally formed with other metals and look like: Elements in period 3 and on can have 5+ bonds (eg. Through that pair, nitrogen can form an. Two core and five valence: I was told that nitrogen can form only up to three bonds. Ad browse & discover thousands of science book titles, for less. Postby andy liao 1b » sun nov 05, 2017 6:53 am. Nitrogen has three electrons in its 2p orbital. Web these four electrons can be gained by forming four covalent bonds, as illustrated here for carbon in ch 4 (methane). Web it has no electrons, which means the nitrogen can donate its lone pair to it, forming a fourth (dative) bond to give nh4 + , the ammonium cation. Web nitrogen has five valence electrons and in simple amines. Either with single, double, or. Atoms of different elements will form either one, two,. Nitrogen has three electrons in its 2p orbital. Web nitrogen is too small to bond to 5 other atoms. Through that pair, nitrogen can form an. Two core and five valence: Either with single, double, or. Web it has no electrons, which means the nitrogen can donate its lone pair to it, forming a fourth (dative) bond to give nh4 + , the ammonium cation. I was told that nitrogen can form only up to three bonds. Group 5a (15) elements such as nitrogen. Elements in period 3 and on can have 5+ bonds (eg. For example, water, (\(\ce{h2o}\)), has two covalent bonds between a single oxygen atom and two. Web if you look at the above image you can see that when nitrogen has a positive charge (one less electron), it can form four covalent bonds. Either with single, double, or. These are. Web nitrogen has five valence electrons and in simple amines it is trivalent, with the two remaining electrons forming a lone pair. Web nitrogen forms four bonds or three? Through that pair, nitrogen can form an. For example, water, (\(\ce{h2o}\)), has two covalent bonds between a single oxygen atom and two. Atoms of different elements will form either one, two,. Web so when nitrogen has four bonds, four bonds and zero lone pairs, zero lone pairs of electrons, we've already seen the formal charge be equal to plus one. Pcl5), but not elements in period 2. Two core and five valence: Atoms of different elements will form either one, two,. Web nitrogen forms four bonds or three? Pcl5), but not elements in period 2. Two core and five valence: Web it has no electrons, which means the nitrogen can donate its lone pair to it, forming a fourth (dative) bond to give nh4 + , the ammonium cation. Group 5a (15) elements such as nitrogen. These are normally formed with other metals and look like: Web it has no electrons, which means the nitrogen can donate its lone pair to it, forming a fourth (dative) bond to give nh4 + , the ammonium cation. Web carbon has six electrons (two core and four valence) and can form four bonds with neighboring atoms. Nitrogen atoms donate four electrons to form n 4 + ions and oxygen. Web nitrogen is too small to bond to 5 other atoms. Either with single, double, or. These are normally formed with other metals and look like: Web if you look at the above image you can see that when nitrogen has a positive charge (one less electron), it can form four covalent bonds. 4 bonds is the most we see. Web nitrogen is in the same group of the periodic table as phosphorus, and you might expect it to form a similar range of compounds. Web these four electrons can be gained by forming four covalent bonds, as illustrated here for carbon in ch 4 (methane). Web if you look at the above image you can see that when nitrogen. Elements in period 3 and on can have 5+ bonds (eg. Web nitrogen (n 2) reacts with oxygen (o 2), the compound n o 2 can be formed as a product. Web these four electrons can be gained by forming four covalent bonds, as illustrated here for carbon in ch 4 (methane). Web two different atoms can also share electrons and form covalent bonds. Pcl5), but not elements in period 2. Nitrogen atoms donate four electrons to form n 4 + ions and oxygen atoms gain two. Web carbon has six electrons (two core and four valence) and can form four bonds with neighboring atoms. Web if you look at the above image you can see that when nitrogen has a positive charge (one less electron), it can form four covalent bonds. Group 5a (15) elements such as nitrogen. Atoms of different elements will form either one, two,. 4 bonds is the most we see nitrogen form. Either with single, double, or. Nitrogen has three electrons in its 2p orbital. Two core and five valence: Web nitrogen has five valence electrons and in simple amines it is trivalent, with the two remaining electrons forming a lone pair. Web when nitrogen forms compounds with other atoms, it primarily forms covalent bonds. Web these four electrons can be gained by forming four covalent bonds, as illustrated here for carbon in ch 4 (methane). Group 5a (15) elements such as nitrogen. Through that pair, nitrogen can form an. Web nitrogen forms four bonds or three?PPT The Nitrogen Cycle PowerPoint Presentation, free download ID899587
PPT Organic compounds containing nitrogen and sulfur PowerPoint
PPT General Chemistry PowerPoint Presentation, free download ID4215385
Why does nitrogen form 4 bonds and oxgen 3 bonds. Can someone explain
Solved How many bonds does nitrogen typically form? Does
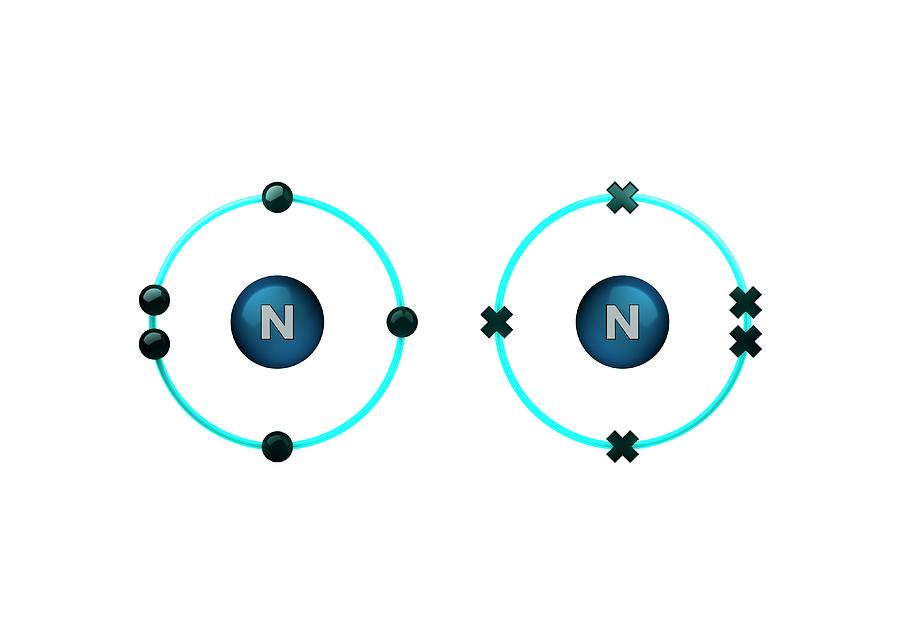
Bond Formation In Nitrogen Molecule Photograph by
LabXchange
PPT Structure and Bonding PowerPoint Presentation, free download ID
PPT COVALENT BONDING PowerPoint Presentation, free download ID5128236
Nitrogen Bonding Exploring How Many Bonds Nitrogen Can Form
Related Post: