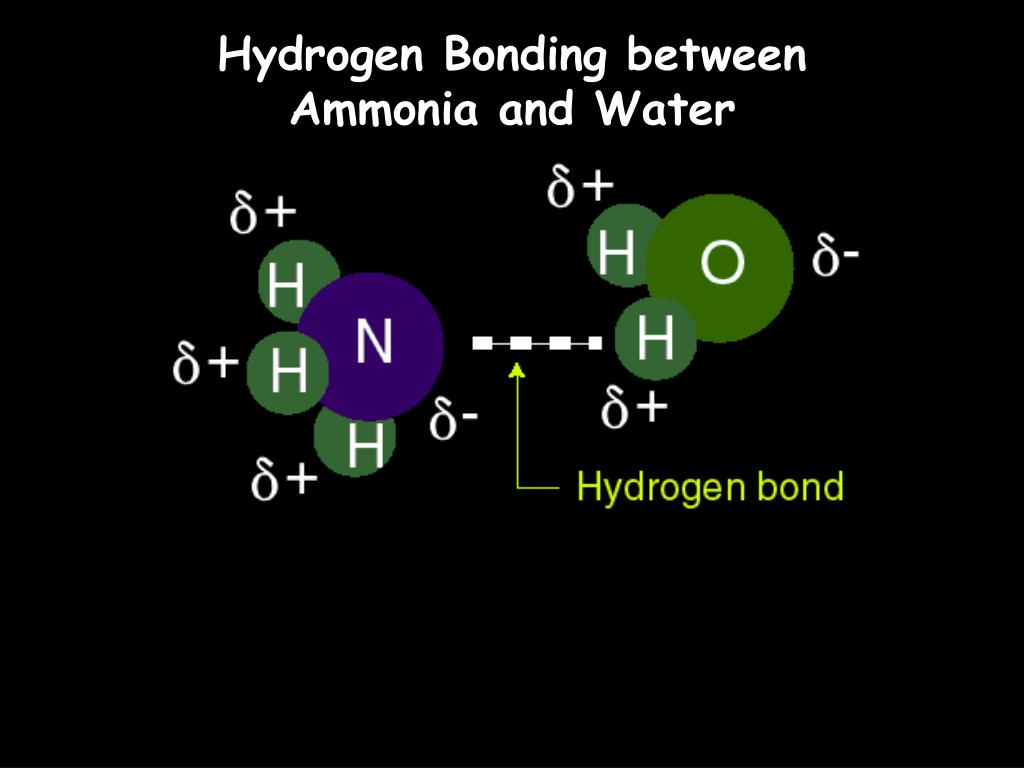
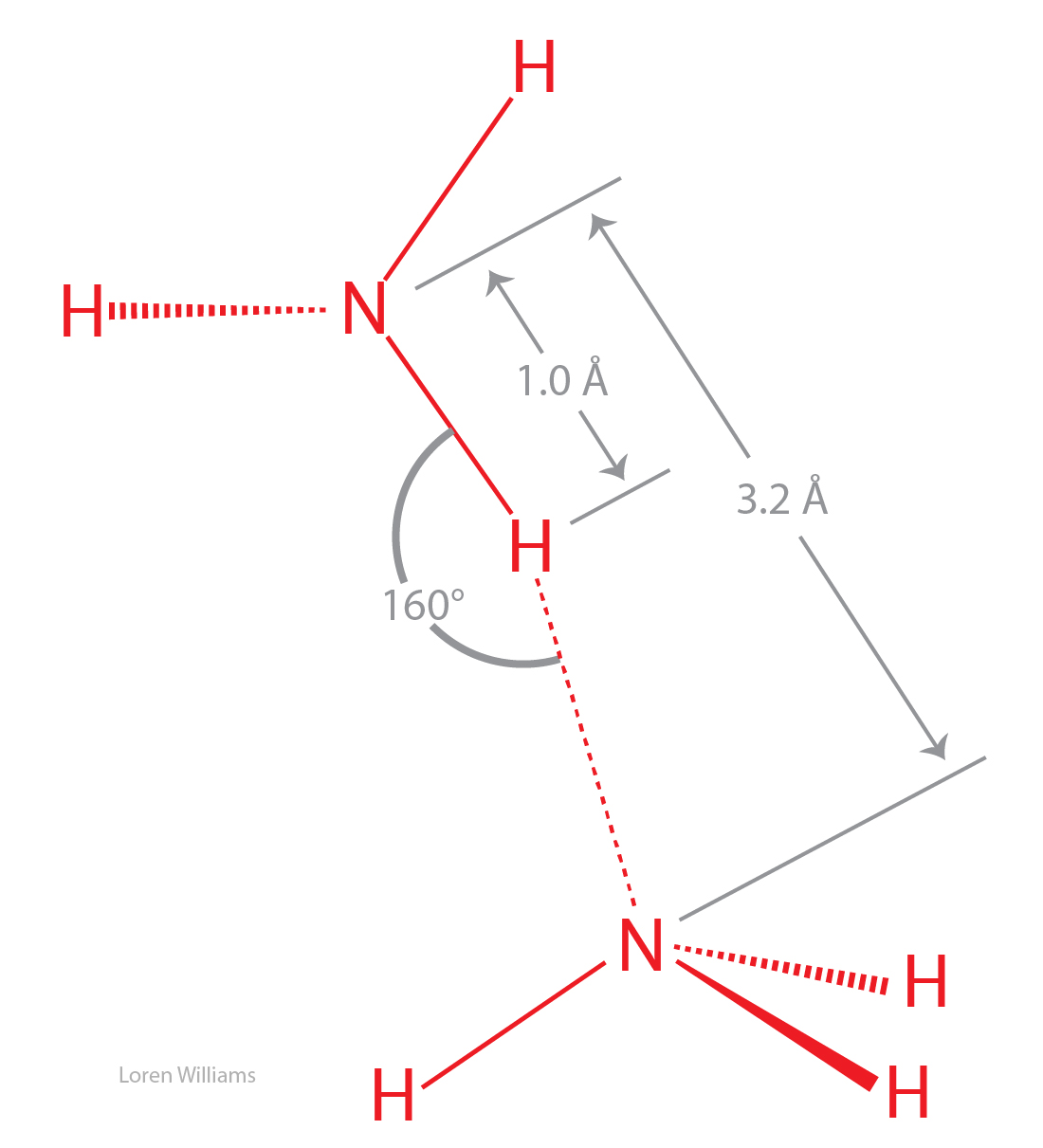
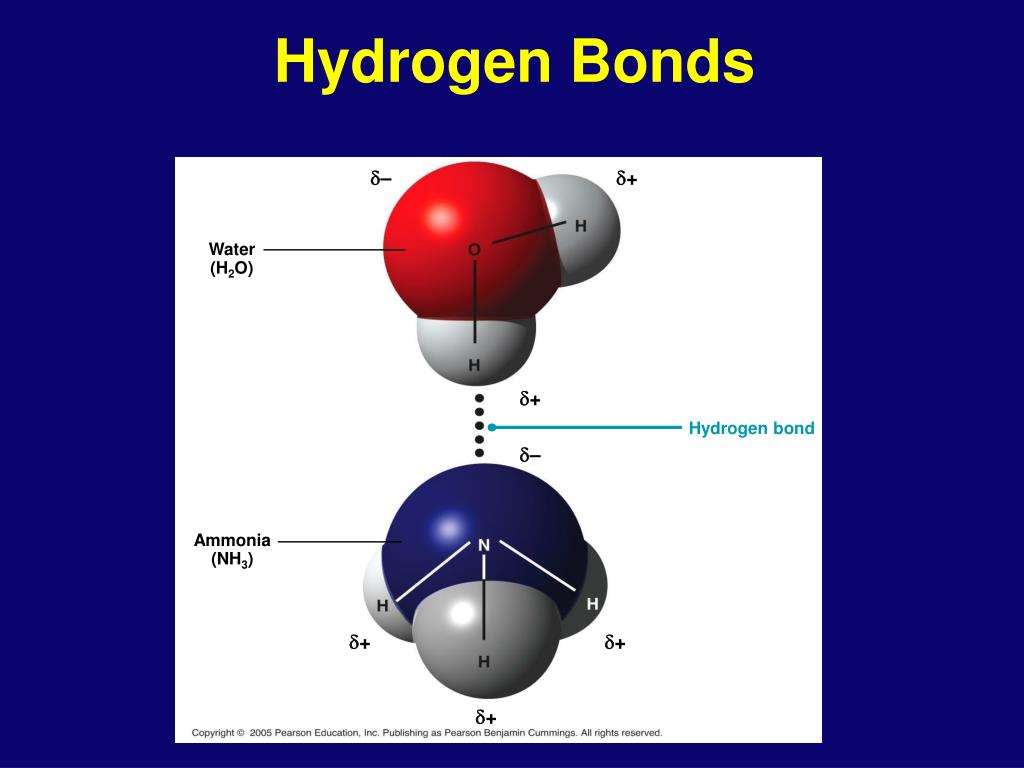
Can Nh3 Form Hydrogen Bonds
Can Nh3 Form Hydrogen Bonds - A) ch4 b) nah c) nh3 d) bh3 e) hi. Web solution verified by toppr n has small atomic size and high electronegativity. Due to the electronegativity difference. Web the hydrogen atoms involved in hydrogen bonding must be attached to electronegative atoms, such as o , n , or f. Web hydrogen bonds are formed in cyclic ammonia clusters, with each ammonia molecule acting simultaneously as a h atom donor and acceptor. P has large size and low electronegativity. Web hexanediol ( ho(ch2)6oh h o ( c h 2) 6 o h) is readily soluble, and if we consider its structure we can see that interactions between hexanediol molecules include hydrogen. Web nh3 3d model: This is because it contains a nitrogen atom (n), which is one of the three atoms (the others being oxygen and fluorine) that can form. Hydrogen bonding is the intermolecular forces acting between ammonia molecules. Web solution verified by toppr n has small atomic size and high electronegativity. It results from the attractive force between a. Nh3 is a chemical compound known as ammonia. Web as expected, nh 3 is observed to be a nearly universal proton acceptor, accepting hydrogen bonds from even some of the weakest proton donors. Web the most powerful intermolecular force. Nh3 is a chemical compound known as ammonia. Web nh3 3d model: Web as expected, nh 3 is observed to be a nearly universal proton acceptor, accepting hydrogen bonds from even some of the weakest proton donors. Web can nh3 form hydrogen bonds? Can nh3 form hydrogen bonds? P has large size and low electronegativity. Web as expected, nh 3 is observed to be a nearly universal proton acceptor, accepting hydrogen bonds from even some of the weakest proton donors. Web nh3 3d model: Web the most powerful intermolecular force influencing neutral (uncharged) molecules is the hydrogen bond. It results from the attractive force between a. Nh3 is a chemical compound known as ammonia. Due to the electronegativity difference. Web chemistry questions and answers. Web hexanediol ( ho(ch2)6oh h o ( c h 2) 6 o h) is readily soluble, and if we consider its structure we can see that interactions between hexanediol molecules include hydrogen. Web the most powerful intermolecular force influencing neutral (uncharged) molecules. A molecule of ammonia can. Due to the electronegativity difference. Web can nh3 form hydrogen bonds? Web the most powerful intermolecular force influencing neutral (uncharged) molecules is the hydrogen bond. 4) which of the following molecules can form hydrogen bonds? Hydrogen bonding is the intermolecular forces acting between ammonia molecules. Web the hydrogen atoms involved in hydrogen bonding must be attached to electronegative atoms, such as o , n , or f. Web as expected, nh 3 is observed to be a nearly universal proton acceptor, accepting hydrogen bonds from even some of the weakest proton donors. Web nh3 3d. It is a gas at room temperature and. Web hydrogen bonds are formed in cyclic ammonia clusters, with each ammonia molecule acting simultaneously as a h atom donor and acceptor. A) ch4 b) nah c) nh3 d) bh3 e) hi. Hence, nh 3 can form hydrogen bonds. If we compare the boiling points of methane (ch 4 ). Water molecules are also attracted to other polar molecules. It is a gas at room temperature and. Due to the electronegativity difference. Web hexanediol ( ho(ch2)6oh h o ( c h 2) 6 o h) is readily soluble, and if we consider its structure we can see that interactions between hexanediol molecules include hydrogen. Web as expected, nh 3 is. Can nh3 form hydrogen bonds? If we compare the boiling points of methane (ch 4 ). Water molecules are also attracted to other polar molecules. Web hexanediol ( ho(ch2)6oh h o ( c h 2) 6 o h) is readily soluble, and if we consider its structure we can see that interactions between hexanediol molecules include hydrogen. Web hydrogen bonds. A molecule of ammonia can. Hydrogen bonding is the intermolecular forces acting between ammonia molecules. Web hydrogen bonds are formed in cyclic ammonia clusters, with each ammonia molecule acting simultaneously as a h atom donor and acceptor. Web yes, nh3 forms hydrogen bonds. Web solution verified by toppr n has small atomic size and high electronegativity. If we compare the boiling points of methane (ch 4 ). Web nh 3 (ammonia) does have hydrogen bonding. Hence, nh 3 can form hydrogen bonds. A) ch4 b) nah c) nh3 d) bh3 e) hi. Web nh3 3d model: Nh3 is a chemical compound known as ammonia. Web we would like to show you a description here but the site won’t allow us. It results from the attractive force between a. Water molecules are also attracted to other polar molecules. This is because it contains a nitrogen atom (n), which is one of the three atoms (the others being oxygen and fluorine) that can form. Due to the electronegativity difference. 4) which of the following molecules can form hydrogen bonds? It is a gas at room temperature and. Web hexanediol ( ho(ch2)6oh h o ( c h 2) 6 o h) is readily soluble, and if we consider its structure we can see that interactions between hexanediol molecules include hydrogen. Web hydrogen bonds are formed in cyclic ammonia clusters, with each ammonia molecule acting simultaneously as a h atom donor and acceptor. Hydrogen bonding is the intermolecular forces acting between ammonia molecules. Web solution verified by toppr n has small atomic size and high electronegativity. Web can nh3 form hydrogen bonds? Web yes, nh3 forms hydrogen bonds. Can nh3 form hydrogen bonds?Does NH3 have Hydrogen Bonding Techiescientist
[Solved] Ammonia, NH3, exhibits hydrogen bonding. Using five molecules
PPT Unit 3 Chemical Bonding and Molecular Structure PowerPoint
chemistry Intermolecular Hydrogen Bonding
Molecular Interactions (Noncovalent Interactions)
Hydrogen Bonding Definition, Example, Types, Question Embibe
How many hydrogen bonds form by Nh3, H20 and HF and boiling point trend
How do Hydrogen bonds form in H2O NH3 HF Hydrogen Bonding
Hydrogen Bonding in Ammonia (NH3) YouTube
PPT Essential Chemistry PowerPoint Presentation, free download ID
Related Post: