Can Hcl Form Hydrogen Bonds
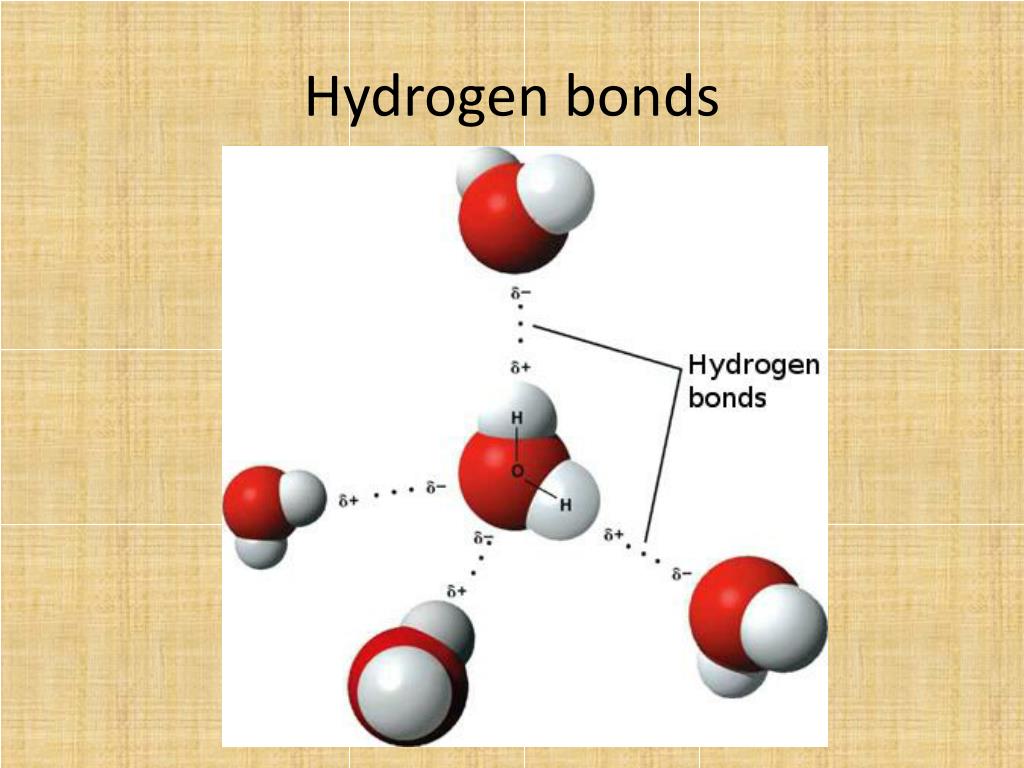
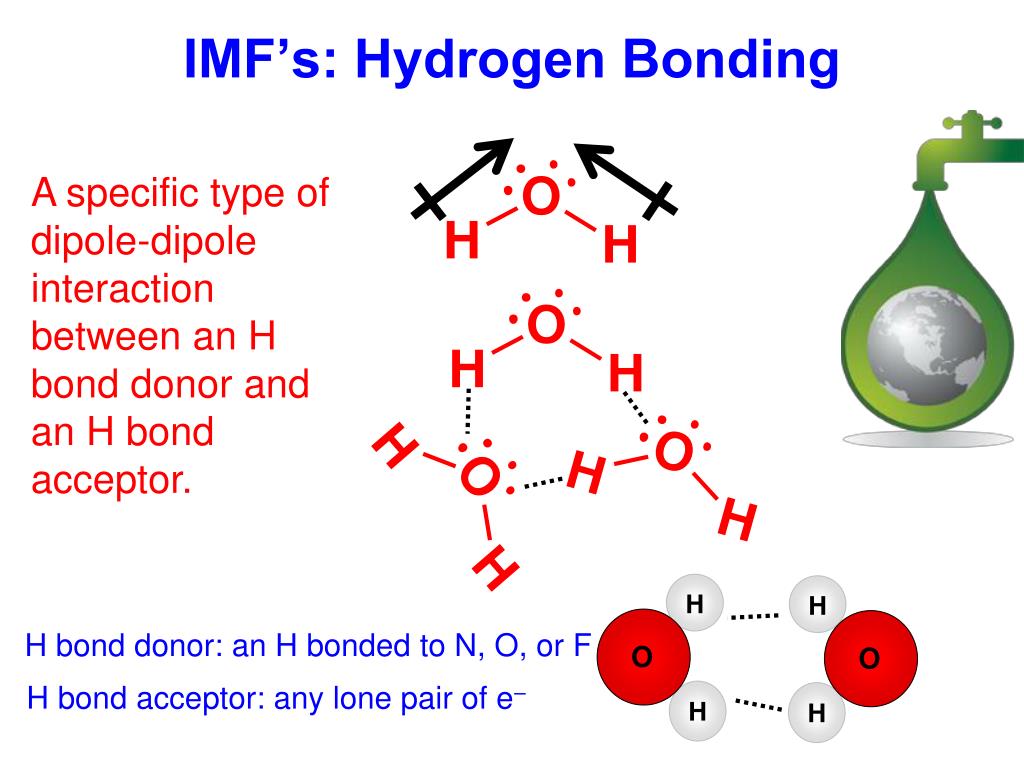
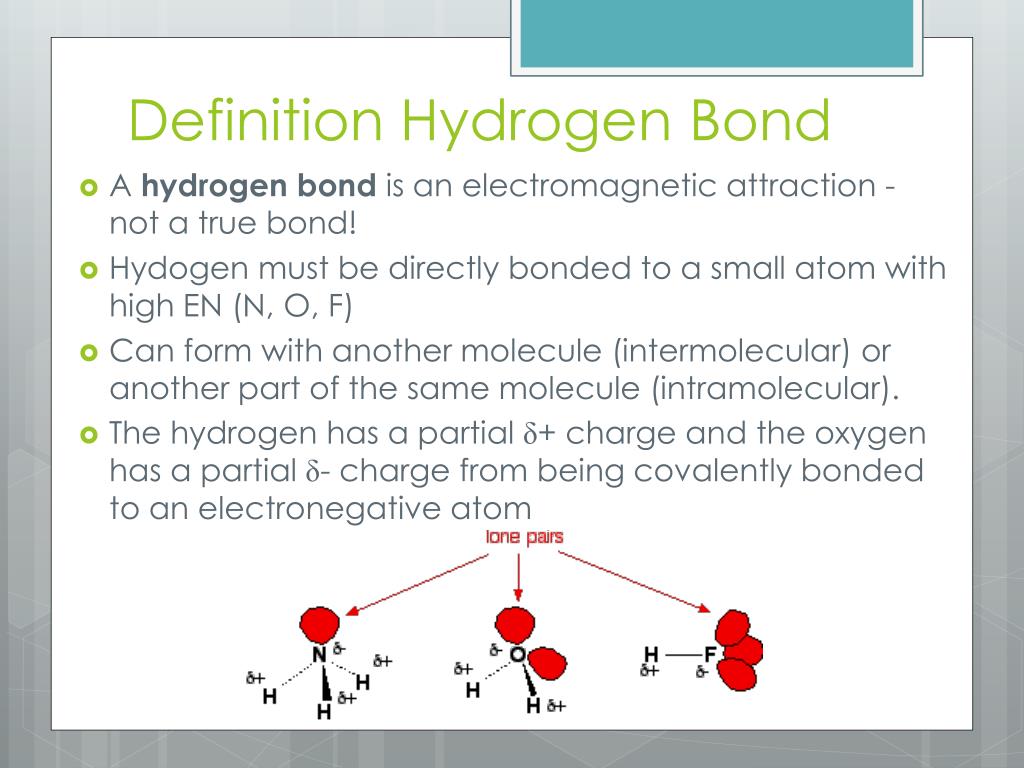
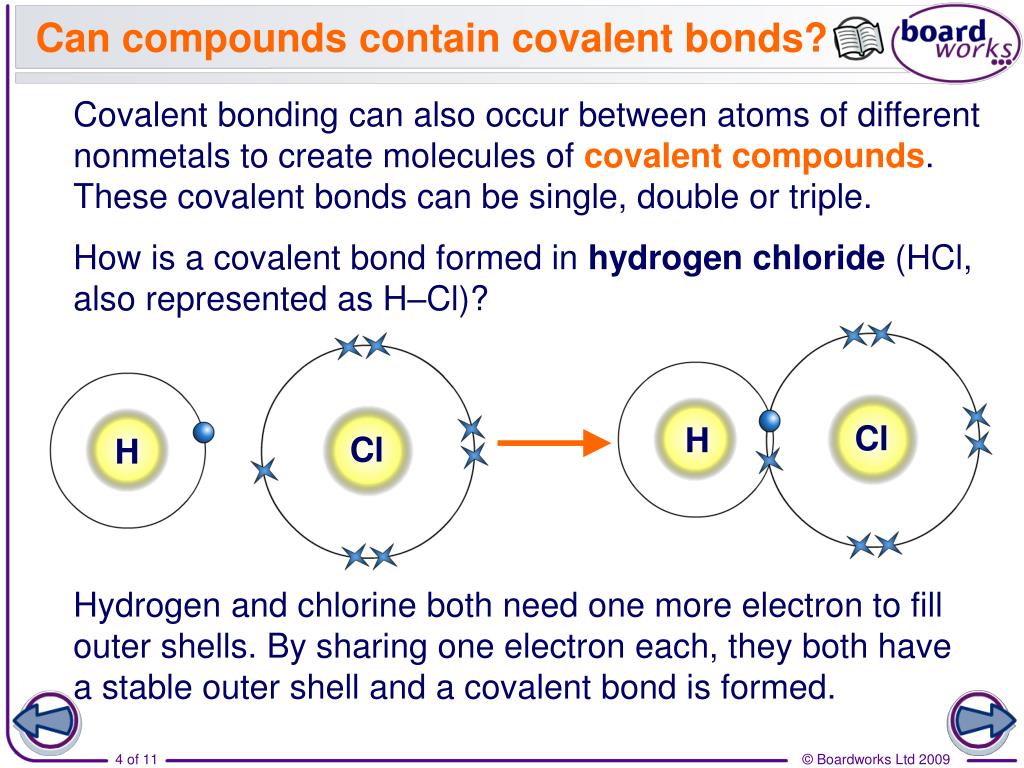
Can Hcl Form Hydrogen Bonds - Web is hcl a hydrogen bond? The chlorine atom is much more. Web hydrogen bonds exist when hydrogen is attached to a fluorine, oxygen, or nitrogen atom. Web the equation for the reaction is the following: These are relatively small atoms that can attach to hydrogen to create a. Web does chlorine form hydrogen bonds? Get deals and low prices on hydrogen bond at amazon H2o + hcl → h3o+ + cl− h 2 o + h c l → h 3 o + + c l −. Web a water molecule consists of two hydrogen atoms bonded to an oxygen atom, and its overall structure is bent. A solution of the gas in water is called. Get deals and low prices on hydrogen bond at amazon Web hydrogen bonds exist when hydrogen is attached to a fluorine, oxygen, or nitrogen atom. These are relatively small atoms that can attach to hydrogen to create a. Web nitrogen is equally electronegative as chlorine but also more compact. Web does chlorine form hydrogen bonds? Even though chlorine is highly electronegative, the best answer is no, and in this class we will consider chlorine not to. Web does chlorine form hydrogen bonds? Web the equation for the reaction is the following: Web hydrogen chloride is a diatomic molecule, consisting of a hydrogen atom h and a chlorine atom cl connected by a polar covalent bond.. Web the equation for the reaction is the following: Hydrogen bonding occurs between hydrogen of one molecule and carbon of another molecule. H2o + hcl → h3o+ + cl− h 2 o + h c l → h 3 o + + c l −. Web chloroform (chcl 3 ): Web is hcl a hydrogen bond? Web which of the following pairs of molecules can form hydrogen bonds between them?a) hcl and hib) ch3oh and nh3c) ch4 and h2od) so2 and ch2oe) h2 and o2 this. A solution of the gas in water is called. Whether you consider hydrogen bonding as purely electrostatic or having a covalent character ( the. H2o + hcl → h3o+ +. The chlorine atom is much more. Even though chlorine is highly electronegative, the best answer is no, and in this class we will consider chlorine not to. Web hexanediol (\(\mathrm{ho}(\mathrm{ch}_{2})_{6}\mathrm{oh}\)) is readily soluble, and if we consider its structure we can see that interactions between hexanediol molecules. Hence, hcl does not have hydrogen bonding. H2o + hcl → h3o+ +. Web the equation for the reaction is the following: The chlorine atom is much more. The h 3 o + ion is the hydroxonium ion (also known as the. Whether you consider hydrogen bonding as purely electrostatic or having a covalent character ( the. The h 3 o + ion is the hydroxonium ion (also known as the hydronium ion. Whether you consider hydrogen bonding as purely electrostatic or having a covalent character ( the. H2o + hcl → h3o+ + cl− h 2 o + h c l → h 3 o + + c l −. Web we would like to show you a description here but the site won’t allow us. Web a water molecule consists of. These are relatively small atoms that can attach to hydrogen to create a. The h 3 o + ion is the hydroxonium ion (also known as the. Hydrogen bonding occurs between hydrogen of one molecule and carbon of another molecule. Web does chlorine form hydrogen bonds? Solution hcl is a covalent bond because when atoms with different electronegativity exchange electrons. H2o + hcl → h3o+ + cl− h 2 o + h c l → h 3 o + + c l −. Web does chlorine form hydrogen bonds? These are relatively small atoms that can attach to hydrogen to create a. Whether you consider hydrogen bonding as purely electrostatic or having a covalent character ( the. Web hydrogen chloride. Web the equation for the reaction is the following: Web hydrogen bonds exist when hydrogen is attached to a fluorine, oxygen, or nitrogen atom. Web nitrogen is equally electronegative as chlorine but also more compact. Get deals and low prices on hydrogen bond at amazon Web is hcl a hydrogen bond? Web a water molecule consists of two hydrogen atoms bonded to an oxygen atom, and its overall structure is bent. Web is hcl a hydrogen bond? Hydrogen bonding occurs between hydrogen of one molecule and carbon of another molecule. Even though chlorine is highly electronegative, the best answer is no, and in this class we will consider chlorine not to. Web which of the following pairs of molecules can form hydrogen bonds between them?a) hcl and hib) ch3oh and nh3c) ch4 and h2od) so2 and ch2oe) h2 and o2 this. Web hexanediol (\(\mathrm{ho}(\mathrm{ch}_{2})_{6}\mathrm{oh}\)) is readily soluble, and if we consider its structure we can see that interactions between hexanediol molecules. Web hydrogen chloride (hcl), a compound of the elements hydrogen and chlorine, a gas at room temperature and pressure. The chlorine atom is much more. Web hydrogen chloride is a diatomic molecule, consisting of a hydrogen atom h and a chlorine atom cl connected by a polar covalent bond. H2o + hcl → h3o+ + cl− h 2 o + h c l → h 3 o + + c l −. Solution hcl is a covalent bond because when atoms with different electronegativity exchange electrons in a covalent link to form a polar covalent. Whether you consider hydrogen bonding as purely electrostatic or having a covalent character ( the. The h 3 o + ion is the hydroxonium ion (also known as the. Web the equation for the reaction is the following: These are relatively small atoms that can attach to hydrogen to create a. Web despite its electronegativity, size of chlorine atom is large and hence, electron density of chlorine is not sufficient to form hydrogen bonding. Web hydrogen bonds exist when hydrogen is attached to a fluorine, oxygen, or nitrogen atom. Web chloroform (chcl 3 ): Get deals and low prices on hydrogen bond at amazon A solution of the gas in water is called.PPT Chemistry of Life PowerPoint Presentation, free download ID2666943
Primary and Secondary Bonds Owlcation
Chapter 5.6 Properties of Polar Covalent Bonds Chemistry LibreTexts
PPT Unit 5B Covalent Bonding PowerPoint Presentation, free download
PPT Hydrogen Bonding PowerPoint Presentation, free download ID3887591
Hydrogen Chloride Molecule Bond Formation Photograph by
HCl Molecular Geometry Science Education and Tutorials
Media Portfolio
PPT Covalent bonding in hydrogen PowerPoint Presentation, free
Chemical Combination Prudent Notes
Related Post: