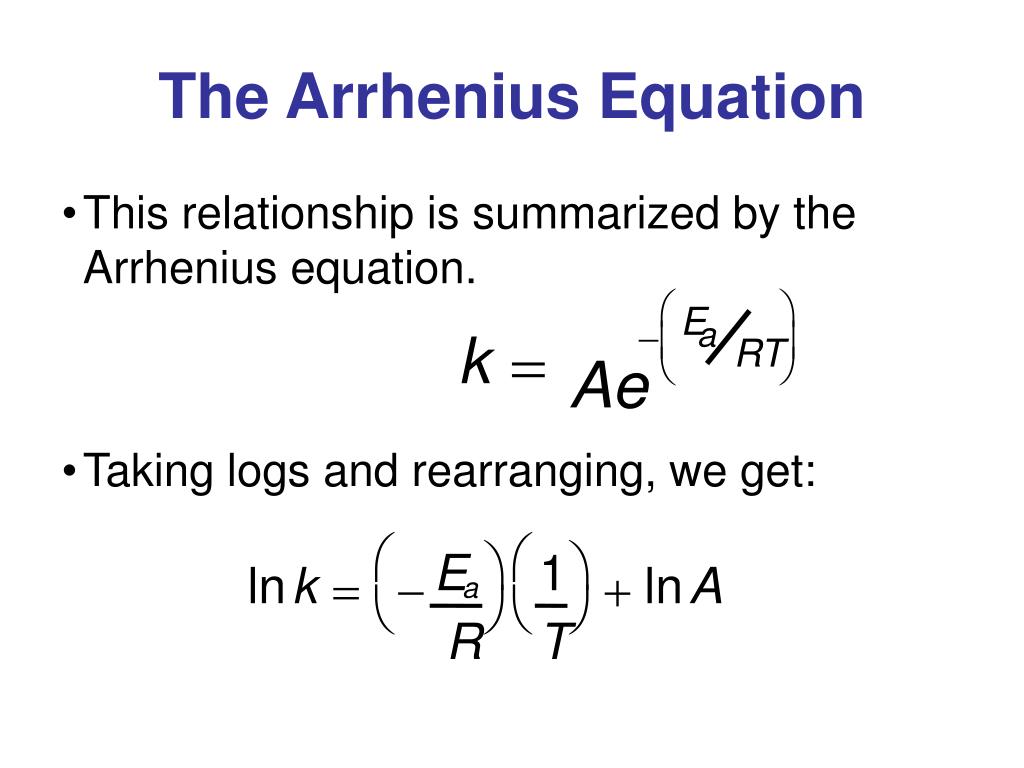
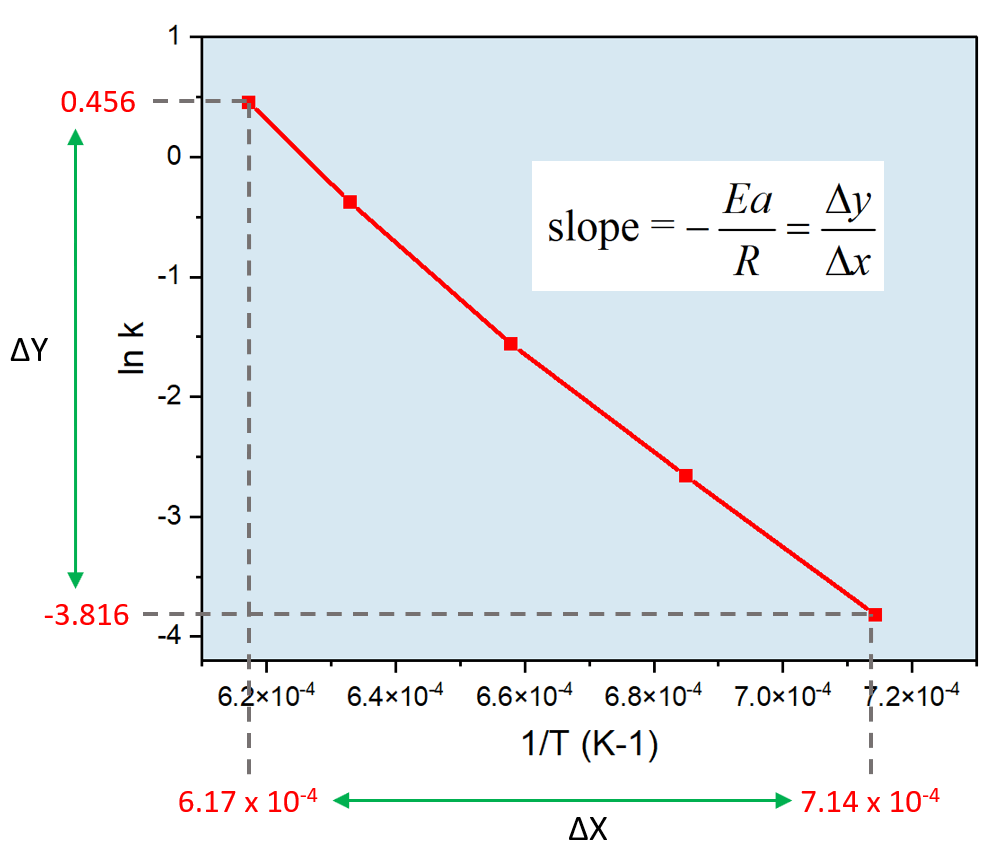
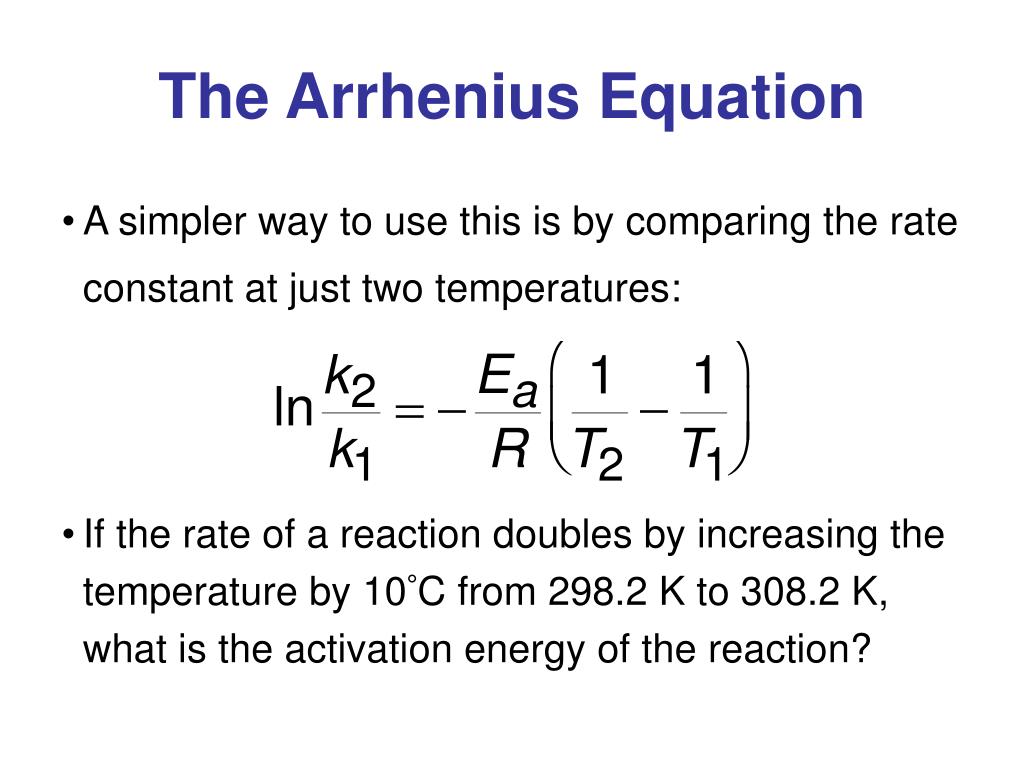
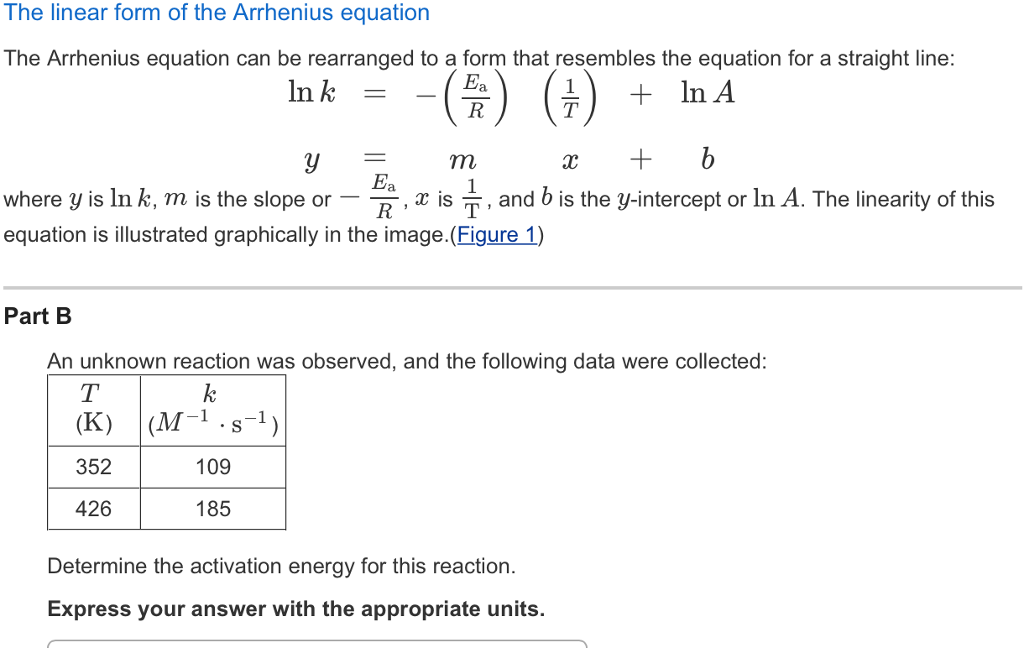
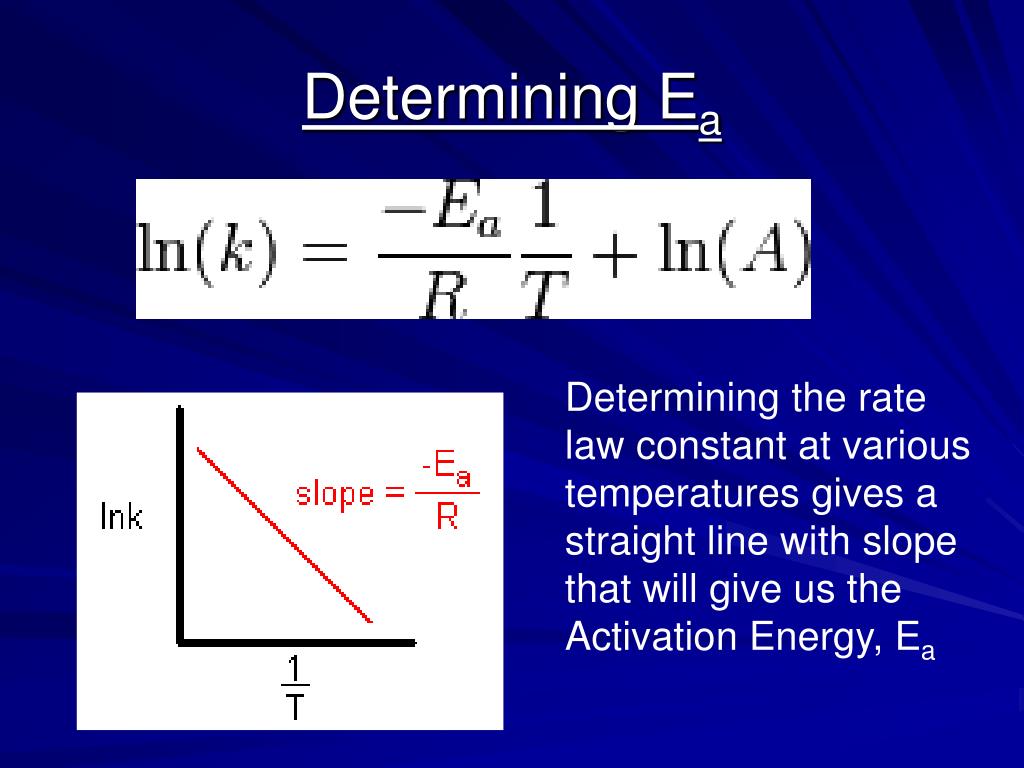
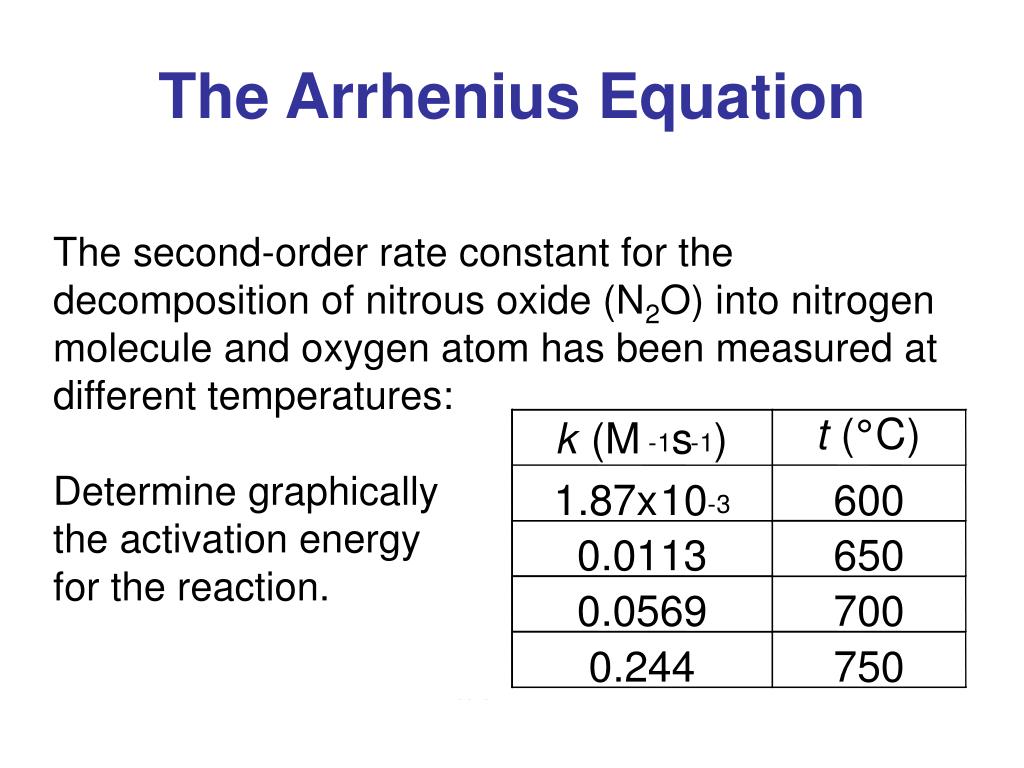
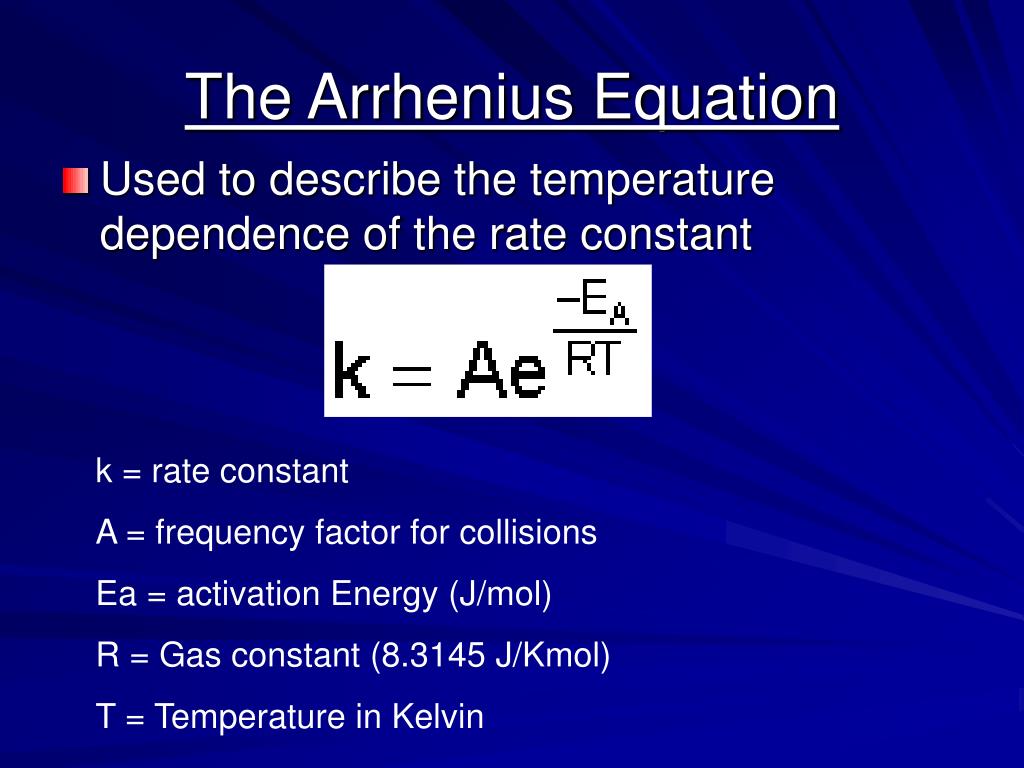
Arrhenius Equation Linear Form
Arrhenius Equation Linear Form - At an absolute temperature t, the fraction of molecules that have a kinetic energy greater than ea can be calculated from statistical mechanics. Estion 7 yet wered nts out of select. Frequency factor, activation energy d. If you need to use this equation, just find the ln button on your calculator. Reaction mechanism and rate law. To relate the activation energy and the rate constant, k, of a given reaction: This means that we're comparing activation. Web the arrhenius equation. Linear form \[\ln k = \dfrac{. Web the linear form of the arrhenius equation the arrhenius equation can be rearranged to a form that resembles the equation for a straight line: Estion 7 yet wered nts out of select. Web the linear form of the arrhenius equation the arrhenius equation can be rearranged to a form that resembles the equation for a straight line: In this equation, r is the ideal gas constant,. Where q is an activation. Web ln is a form of logarithm. Frequency factor, activation energy d. Web the arrhenius equation is in linear form if we graph ln(kr) l n ( k r) as y and 1 t 1 t as x: Arrhenius argued that for reactants to transform into products, they must first acquire a minimum amount of energy, called the activation energy ea. Web arrhenius equation, mathematical expression that. Temperature, t, measured in kelvin. Frequency factor, activation energy d. Web ln is a form of logarithm. Where q is an activation. In this equation, r is the ideal gas constant,. Web the linear form of the arrhenius equation is very useful, as it allows us to calculate the from the slope and the from the intercept. Forms of the arrhenius equation. Web equation \(\ref{4}\) has the linear form y = mx + b. To relate the activation energy and the rate constant, k, of a given reaction: Web the arrhenius. Don't worry about what it means. Estion 7 yet wered nts out of select. Arrehenius equation in linear form. K = ae−ea/rt k = a e − e a / r t. Web this is shown mathematically in the arrhenius equation: Frequency factor, activation energy d. Web ln is a form of logarithm. This means that we're comparing activation. Web the arrhenius equation. Forms of the arrhenius equation. The linear form of the arrhenius equation the arrhenius equation can be rearranged to a form that resembles the equation for a straight line: Frequency factor, activation energy d. Web ln is a form of logarithm. If you need to use this equation, just find the ln button on your calculator. To relate the activation energy and the rate constant,. Linear form \[\ln k = \dfrac{. At an absolute temperature t, the fraction of molecules that have a kinetic energy greater than ea can be calculated from statistical mechanics. When dealing with two rate constants or two temperatures then we. The various symbols represent the following: Temperature, t, measured in kelvin. Ln(kr)= −ea r × 1 t +ln(a) l n ( k r) = − e a r × 1 t + l n ( a) where. Web equation \(\ref{4}\) has the linear form y = mx + b. At an absolute temperature t, the fraction of molecules that have a kinetic energy greater than ea can be calculated from statistical. At an absolute temperature t, the fraction of molecules that have a kinetic energy greater than ea can be calculated from statistical mechanics. Linear form \[\ln k = \dfrac{. The various symbols represent the following: In this equation, r is the ideal gas constant,. If you need to use this equation, just find the ln button on your calculator. The linear form of the arrhenius equation the arrhenius equation can be rearranged to a form that resembles the equation for a straight line: Web equation \(\ref{4}\) has the linear form y = mx + b. Web ln is a form of logarithm. This means that we're comparing activation. Linear form \[\ln k = \dfrac{. If you need to use this equation, just find the ln button on your calculator. Web arrhenius equation, mathematical expression that describes the effect of temperature on the velocity of a chemical reaction, the basis of all expressions used for. Web this is shown mathematically in the arrhenius equation: Frequency factor, activation energy d. The concept of activation energy explains the exponential nature of the relationship, and in one way or another, it is present in all kinetic theories. Web the arrhenius equation is in linear form if we graph ln(kr) l n ( k r) as y and 1 t 1 t as x: K = ae−ea/rt k = a e − e a / r t. Arrhenius argued that for reactants to transform into products, they must first acquire a minimum amount of energy, called the activation energy ea. Temperature, t, measured in kelvin. When dealing with two rate constants or two temperatures then we. Estion 7 yet wered nts out of select. Where q is an activation. Reaction mechanism and rate law. Web the arrhenius equation. In this equation, r is the ideal gas constant,.PPT The Arrhenius Equation PowerPoint Presentation, free download
Arrhenius Equation Chemistry Steps
PPT The Arrhenius Equation PowerPoint Presentation, free download
Solved The linear form of the Arrhenius equation The
The other forms of the Arrhenius Equation YouTube
The Arrhenius Equation (A Level Chemistry) Teaching Resources
PPT The Arrhenius Equation PowerPoint Presentation, free download
PPT The Arrhenius Equation PowerPoint Presentation, free download
PPT The Arrhenius Equation PowerPoint Presentation, free download
PPT The Arrhenius Equation PowerPoint Presentation, free download
Related Post: