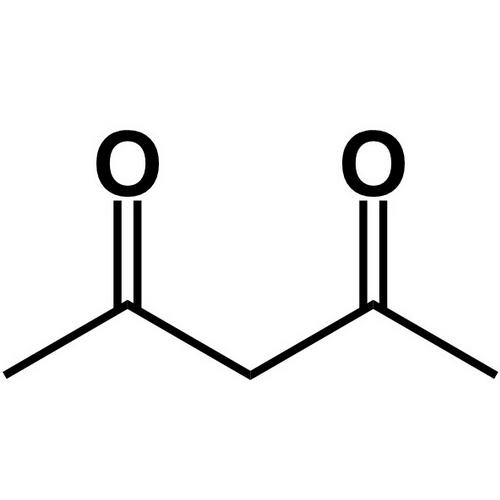
2 4 Pentanedione Enol Form
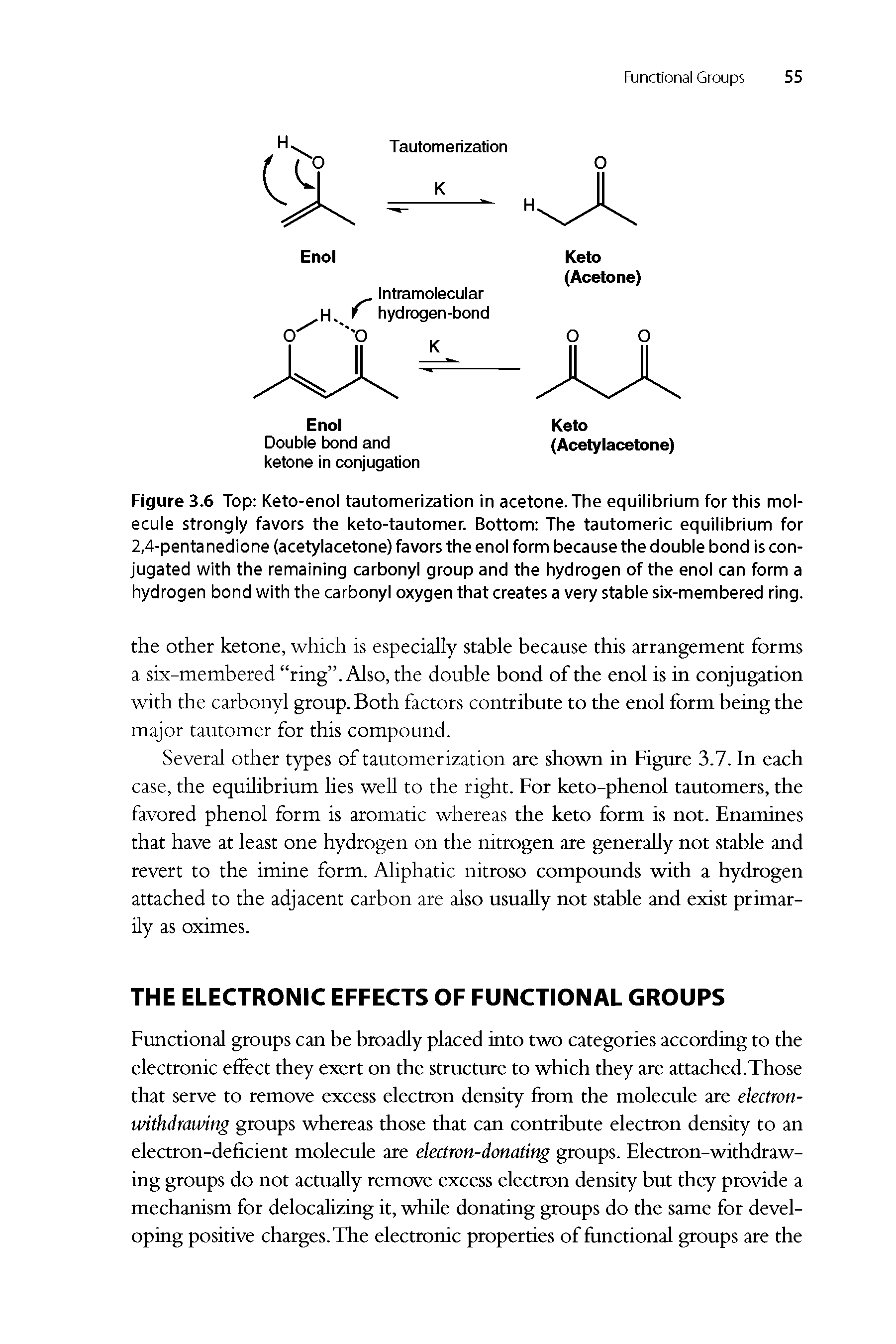
2 4 Pentanedione Enol Form - The two tautomeric forms can be distinguished by nmr spectroscopy, ir spectroscopy and other methods. In this experiment, the student monitors. Web web hydrates lose water to form an enol, which tautomerizes back to the carbonyl compound. [ ə¦sed·əl′as·ə‚tōn] (organic chemistry) ch 3 coch 2 occh 3 a colorless liquid with a pleasant odor and a boiling point of 140.5°c; Web hydrates lose water to form an enol, which tautomerizes back to the carbonyl compound. Because tautomers involve the rearrangement. It is known that most ketones exist in >99% as the keto form. 100% (3 ratings) transcribed image text: When showing the reaction with the iodoform test what would. Although enols are scarce at equilibrium, they are nevertheless responsible for much of the chemistry of carbonyl. It is known that most ketones exist in >99% as the keto form. Draw the structure of the enol form that explains this high percentage. The enol form has c2v symmetry, meaning the hydrogen atom is shared equally between the two oxygen atoms. [ ə¦sed·əl′as·ə‚tōn] (organic chemistry) ch 3 coch 2 occh 3 a colorless liquid with a pleasant odor. The enol form has c2v symmetry, meaning the hydrogen atom is shared equally between the two oxygen atoms. The two tautomeric forms can be distinguished by nmr spectroscopy, ir spectroscopy and other methods. Find related products, papers, technical documents,. Draw the structure of the enol form that explains this high percentage. Because tautomers involve the rearrangement. The enol form has c2v symmetry, meaning the hydrogen atom is shared equally between the two oxygen atoms. Web hydrates lose water to form an enol, which tautomerizes back to the carbonyl compound. Tautomers are readily interconverted constitutional isomers, usually distinguished by a different location for an atom or a group. [ ə¦sed·əl′as·ə‚tōn] (organic chemistry) ch 3 coch 2 occh. The keto and enol tautomers of acetylacetone coexist in solution. The enol form has c2v symmetry, meaning the hydrogen atom is shared equally between the two oxygen atoms. The two center enol structures are in fast exchange in this experiment,. Web hydrates lose water to form an enol, which tautomerizes back to the carbonyl compound. It is known that most. Draw the structure of the enol form that explains this high percentage. The two tautomeric forms can be distinguished by nmr spectroscopy, ir spectroscopy and other methods. It exists in equilibrium with a tautomer. Find related products, papers, technical documents,. 100% (3 ratings) transcribed image text: The two center enol structures are in. It is also known that 2,4. The keto and enol tautomers of acetylacetone coexist in solution. When showing the reaction with the iodoform test what would. In this experiment, the student monitors. The two tautomeric forms can be distinguished by nmr spectroscopy, ir spectroscopy and other methods. The two center enol structures are in fast exchange in this experiment,. [ ə¦sed·əl′as·ə‚tōn] (organic chemistry) ch 3 coch 2 occh 3 a colorless liquid with a pleasant odor and a boiling point of 140.5°c; Although enols are scarce at equilibrium, they are nevertheless responsible. It is known that most ketones exist in >99% as the keto form. The enol form has c2v symmetry, meaning the hydrogen atom is shared equally between the two oxygen atoms. Because tautomers involve the rearrangement. 100% (3 ratings) transcribed image text: Although enols are scarce at equilibrium, they are nevertheless responsible for much of the chemistry of carbonyl. The enol form has c2v symmetry, meaning the hydrogen atom is shared equally between the two oxygen atoms. Web hydrates lose water to form an enol, which tautomerizes back to the carbonyl compound. Although enols are scarce at equilibrium, they are nevertheless responsible for much of the chemistry of carbonyl. In the gas phase, the equilibrium constant, kketo→enol, is 11.7,. In this experiment, the student monitors. Draw the structure of the enol form that explains this high percentage. The two tautomeric forms can be distinguished by nmr spectroscopy, ir spectroscopy and other methods. It is known that most ketones exist in >99% as the keto form. The keto and enol tautomers of acetylacetone coexist in solution. The two center enol structures are in fast exchange in this experiment,. It is also known that 2,4. Because tautomers involve the rearrangement. Although enols are scarce at equilibrium, they are nevertheless responsible for much of the chemistry of carbonyl. The two center enol structures are in. Draw the structure of the enol form that explains this high percentage. The keto and enol tautomers of acetylacetone coexist in solution. The two tautomeric forms can be distinguished by nmr spectroscopy, ir spectroscopy and other methods. Web hydrates lose water to form an enol, which tautomerizes back to the carbonyl compound. 100% (3 ratings) transcribed image text: It is known that most ketones exist in >99% as the keto form. Find related products, papers, technical documents,. In the gas phase, the equilibrium constant, kketo→enol, is 11.7, favoring the enol form. [ ə¦sed·əl′as·ə‚tōn] (organic chemistry) ch 3 coch 2 occh 3 a colorless liquid with a pleasant odor and a boiling point of 140.5°c; The enol form has c2v symmetry, meaning the hydrogen atom is shared equally between the two oxygen atoms. In this experiment, the student monitors. Web web hydrates lose water to form an enol, which tautomerizes back to the carbonyl compound. Tautomers are readily interconverted constitutional isomers, usually distinguished by a different location for an atom or a group. It exists in equilibrium with a tautomer. When showing the reaction with the iodoform test what would.Explain why 2,4pentanedione contains much less enol form i Quizlet
The most stable enolic from of 2, 4pentanedione is YouTube
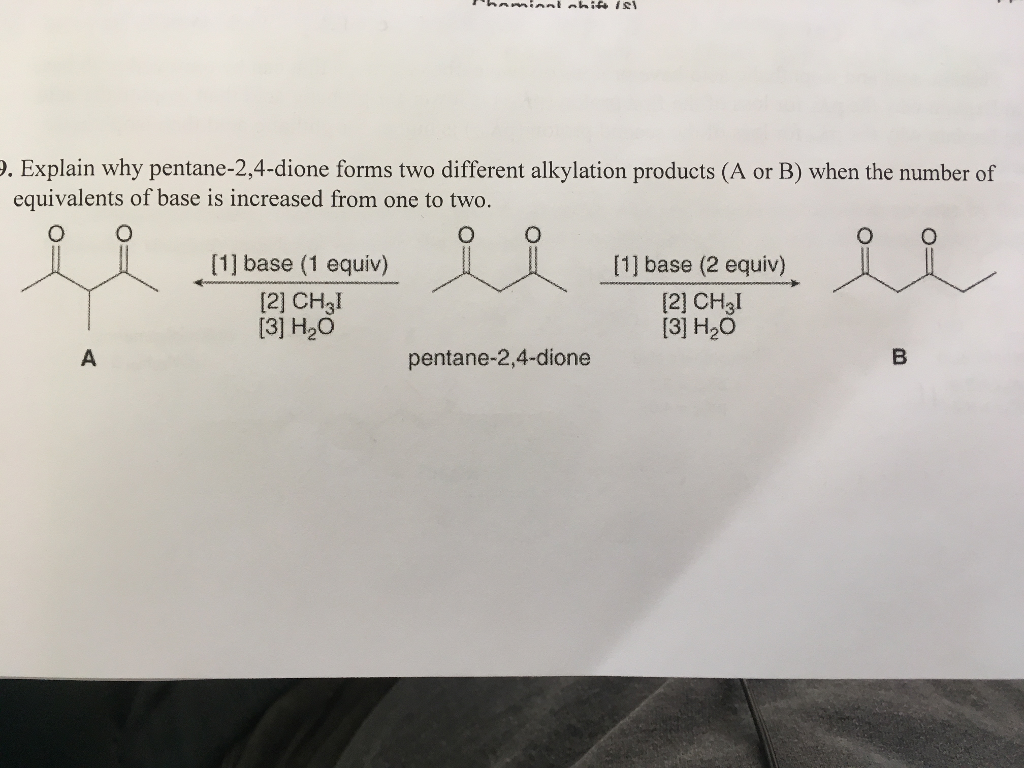
Solved Explain why pentane2, 4dione forms two different
organic chemistry What is the most stable structure amongst the keto
2,4Pentanedione ketoenol equilibrium Big Chemical Encyclopedia
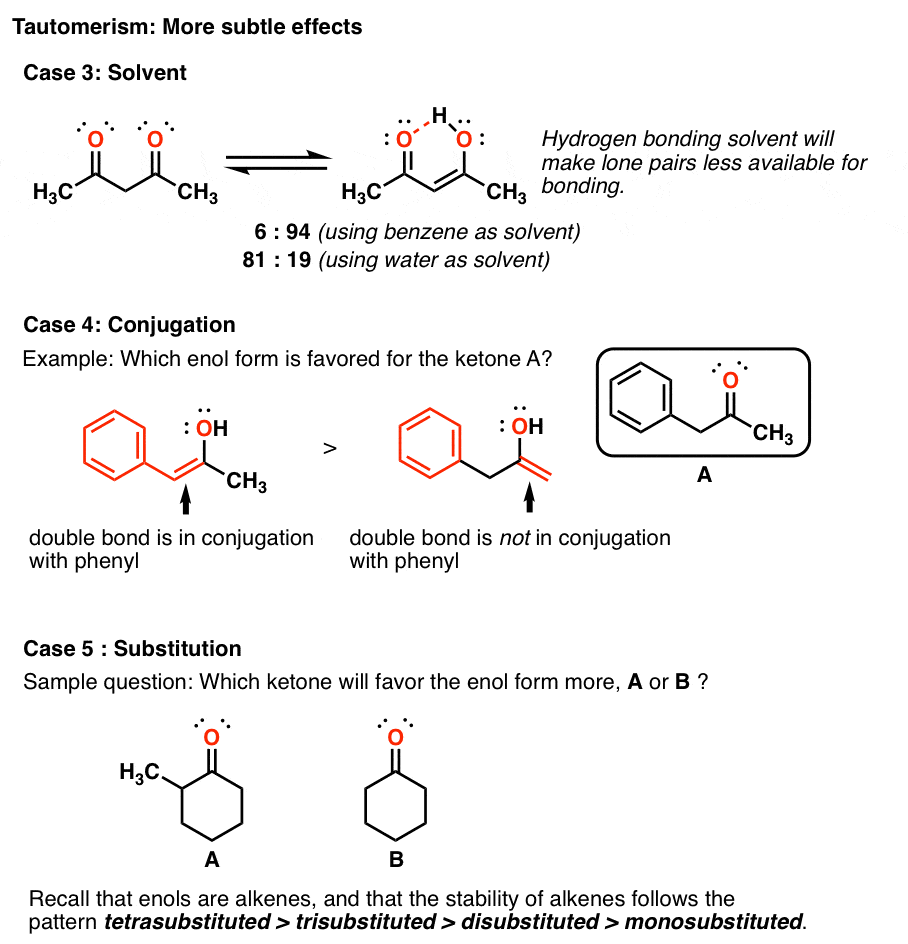
KetoEnol Tautomerism Key Points Master Organic Chemistry
2,4Pentanedione, 99, Alfa Aesar™ Organic Building Blocks Chemicals
PPT Chapter 18 Enols and Enolates PowerPoint Presentation, free
2,4Pentanedione 13C EPTES
the 1 hnmr1 h nmr spectrum 24 pentanedione indicates the presence enol
Related Post: