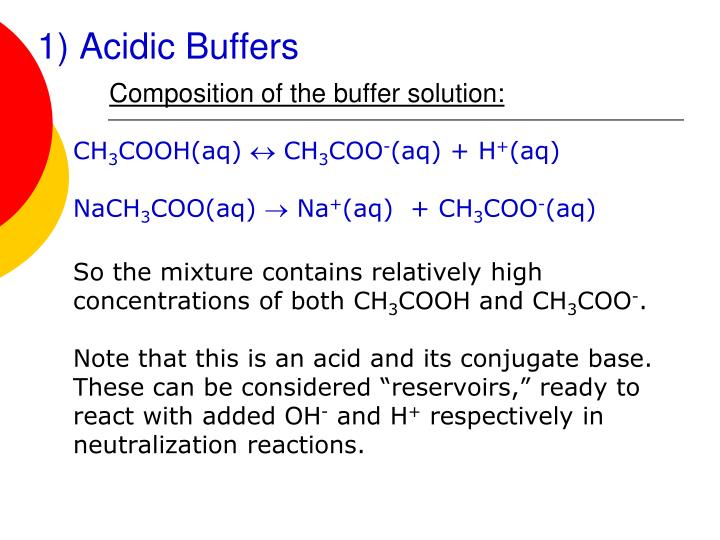
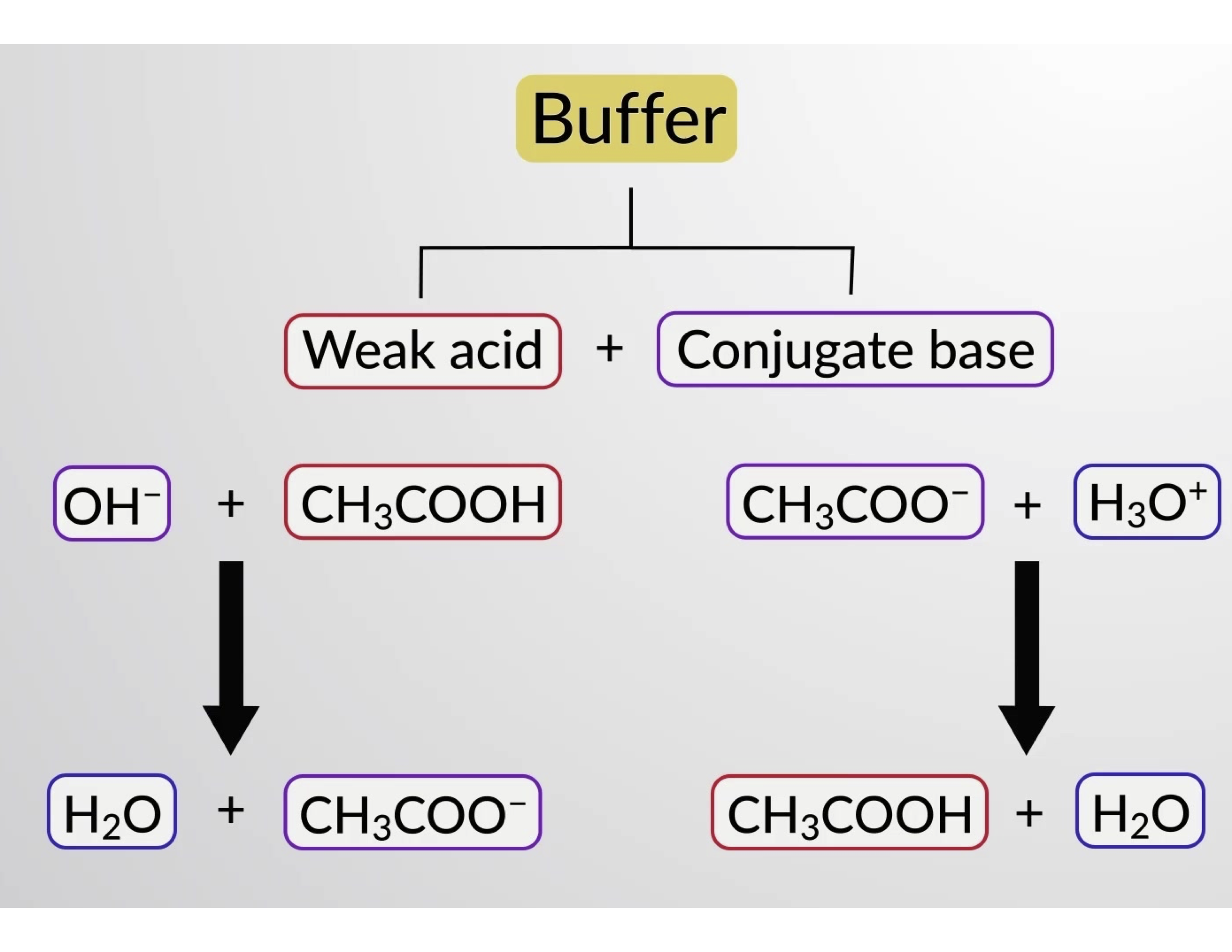
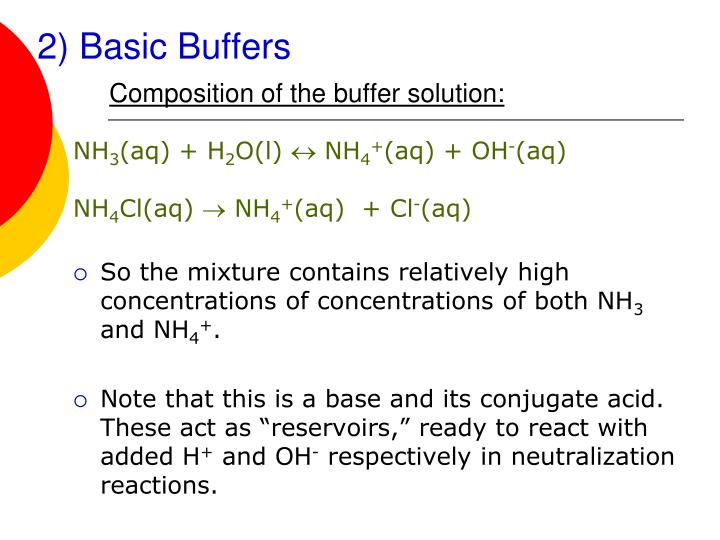
Will Hcl And Nacl Form A Buffer
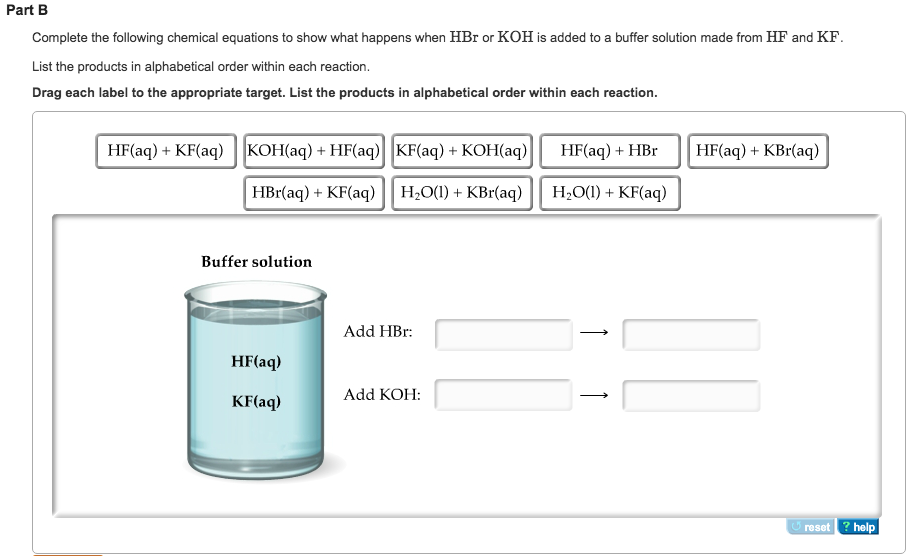
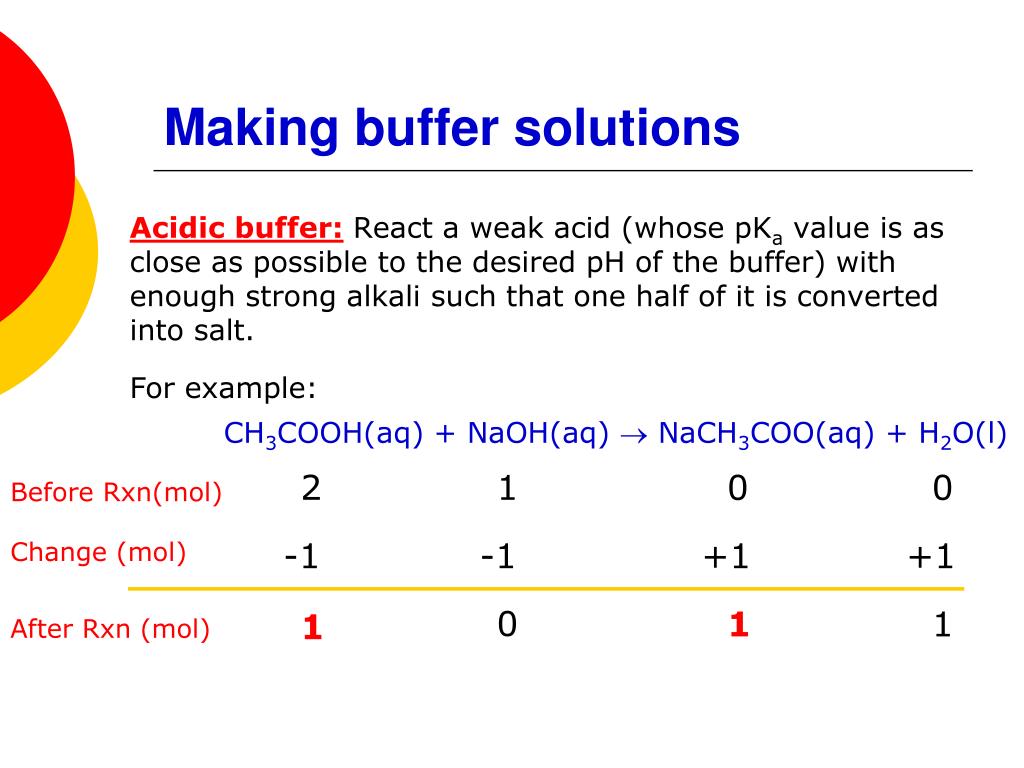
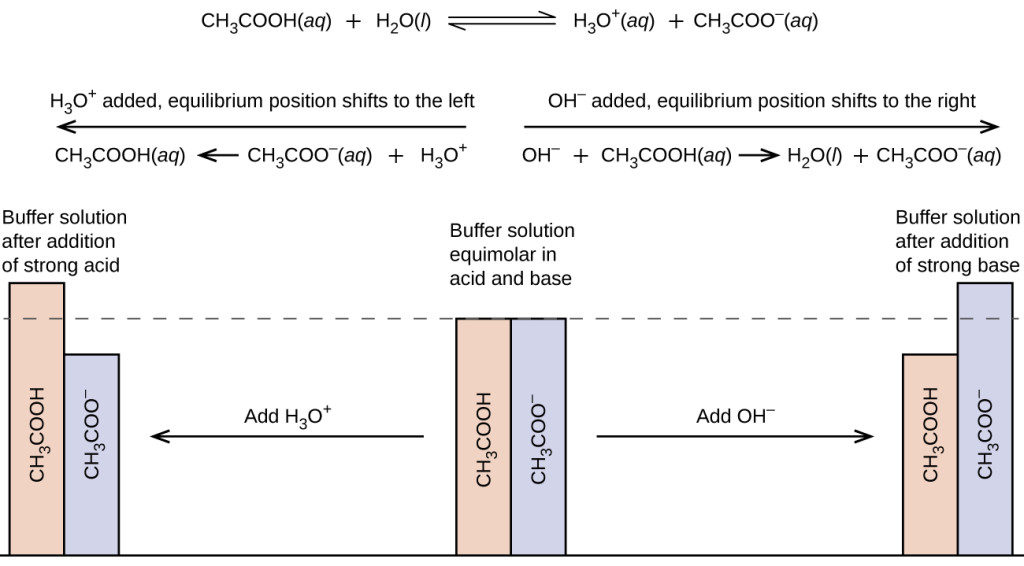
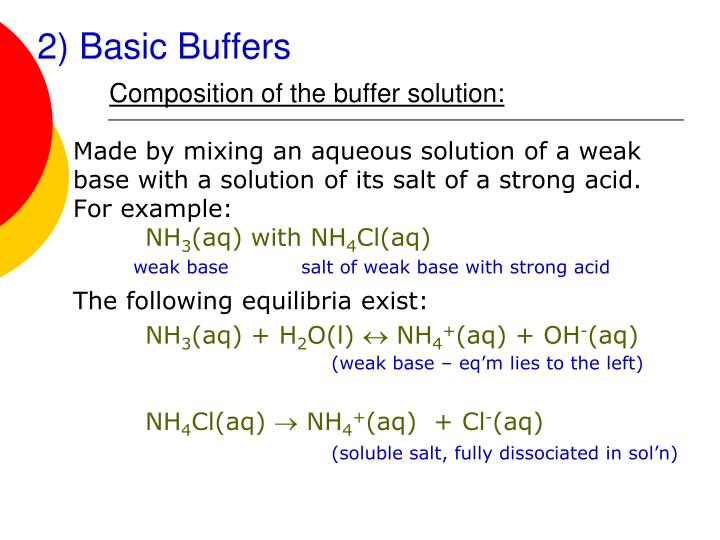
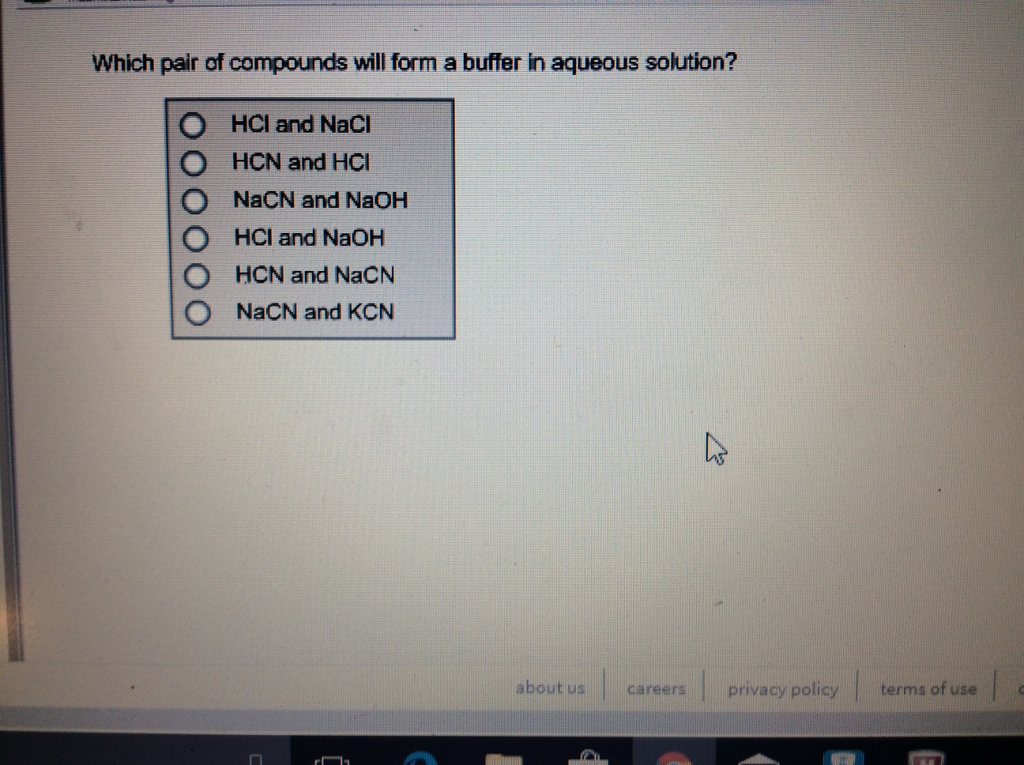
Will Hcl And Nacl Form A Buffer - Web a solution made from nacl and hcl will act as a buffer solution. Web if the solution is not a buffer, the hcl will react with the solution to form a product. Buffers play a crucial role in maintaining the ph balance in various chemical processes. Web hydrochloric acid (hcl) is a strong acid, not a weak acid, so the combination of these two solutes would not make a buffer solution. Assume all are aqueous solutions. Hydrochloric acid (hcl) is a strong acid and its conjugate base is the chloride anion provided from. Even though the second condition for a buffer solution is fulfilled, hcl as a. Hcl is a strong acid and cannot form buffers. Determine which of the following compounds would form a buffer solution when dissolved in water: Web science chemistry chemistry questions and answers 4. Which pair of compounds will form a buffer in aqueous solution? Ch 3 nh 2 and ch 3 nh 3 cl; Web chemistry chemistry questions and answers which pair of compounds will form a buffer in aqueous solution? Even though the second condition for a buffer solution is fulfilled, hcl as a. Web chemistry chemistry questions and answers which pair. Ch 3 nh 2 and ch 3 nh 3 cl; Web chemistry chemistry questions and answers which pair of compounds will form a buffer in aqueous solution? Web why is hcl and nacl not a buffer? Hydrochloric acid (hcl) is a strong acid and its conjugate base is the chloride anion provided from. Web if the solution is not a. Web hydrochloric acid (hcl) is a strong acid, not a weak acid, so the combination of these two solutes would not make a buffer solution. Web we would like to show you a description here but the site won’t allow us. The solution made from nacl and hcl will not act as a buffer. Web if the solution is not. Naoh + hcl → h 2 o and nacl. Assume all are aqueous solutions. Web hydrochloric acid (hcl) is a strong acid, not a weak acid, so the combination of these two solutes would not make a buffer solution. Web why is hcl and nacl not a buffer? Even though the second condition for a buffer solution is fulfilled, hcl. Which pair of compounds will form a buffer in aqueous solution? Web why is hcl and nacl not a buffer? Naoh + hcl → h 2 o and nacl. While hydrochloric acid (hcl) and sodium chloride (nacl). Hydrochloric acid (hcl) is a strong acid and its conjugate base is the chloride anion provided from. A buffer system is used to control the ph of an aqueous solution in a certain narrow range. Web chemistry chemistry questions and answers which pair of compounds will form a buffer in aqueous solution? Hydrochloric acid (hcl) is a strong acid and its conjugate base is the chloride anion provided from. Web for example, a buffer can be composed. The overall equation for this reaction is: Web science chemistry chemistry questions and answers 4. Web how does nacl react with hcl? A buffer system is used to control the ph of an aqueous solution in a certain narrow range. Positive hydrogen ions from hcl and negative hydroxide ions from naoh. Hcl is a strong acid and cannot form buffers. Web how does nacl react with hcl? Buffers play a crucial role in maintaining the ph balance in various chemical processes. Ch 3 nh 2 and ch 3 nh 3 cl; A) hcl and naoh b) hcl and naci c) hcn and nacn d) hcn and hci e) nacn and naoh. Hydrochloric acid (hcl) is a strong acid and its conjugate base is the chloride anion provided from. Web we would like to show you a description here but the site won’t allow us. Hcl and naoh hcn and hcl hcl and nacl hcn and nacn nacn. Web for example, a buffer can be composed of dissolved hc 2 h 3. Web hcl and nacl as a buffer. Web hydrochloric acid (hcl) is a strong acid, not a weak acid, so the combination of these two solutes would not make a buffer solution. Hcl is a strong acid and cannot form buffers. Web chrome_reader_mode input reader mode. Web hcl and nacl are not buffer solutions. The solution made from nacl and hcl will not act as a buffer. Web will hcl and nacl form a buffer in aqueous solution? Web chemistry chemistry questions and answers which pair of compounds will form a buffer in aqueous solution? Web hcl and nacl are not buffer solutions. Web why is hcl and nacl not a buffer? A) hcl and naoh b) hcl and naci c) hcn and nacn d) hcn and hci e) nacn and naoh f) nacn. Web for example, a buffer can be composed of dissolved hc 2 h 3 o 2 (a weak acid) and nac 2 h 3 o 2 (the salt derived from that weak acid). Web which solute combinations can make a buffer solution? Web hydrochloric acid (hcl) is a strong acid, not a weak acid, so the combination of these two solutes would not make a buffer solution. Even though the second condition for a buffer solution is fulfilled, hcl as a. A buffer system is used to control the ph of an aqueous solution in a certain narrow range. Hcl is a strong acid and cannot form buffers. Web example 15 which combinations of compounds can make a buffer solution? Which pair of compounds will form a buffer in aqueous solution? Positive hydrogen ions from hcl and negative hydroxide ions from naoh. Buffers play a crucial role in maintaining the ph balance in various chemical processes. Hcl and naoh hcn and hcl hcl and nacl hcn and nacn nacn. Web a solution made from nacl and hcl will act as a buffer solution. Hcho 2 and nacho 2 hcl and nacl ch 3 nh 2 and ch 3 nh 3 cl nh 3 and naoh solution hcho. Determine which of the following compounds would form a buffer solution when dissolved in water:Solved Part A Which Set Of Compounds Would Form A Buffer
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint
Which Pair Will Produce a Buffer Solution AllysonhasMccoy
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint
Which pair of compounds will form a buffer in an aqueous solution? a
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint
Buffers, Buffer Components and Buffer Action Chemistry JoVE
Solved Which pair of compounds will form a buffer in aqueous
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint
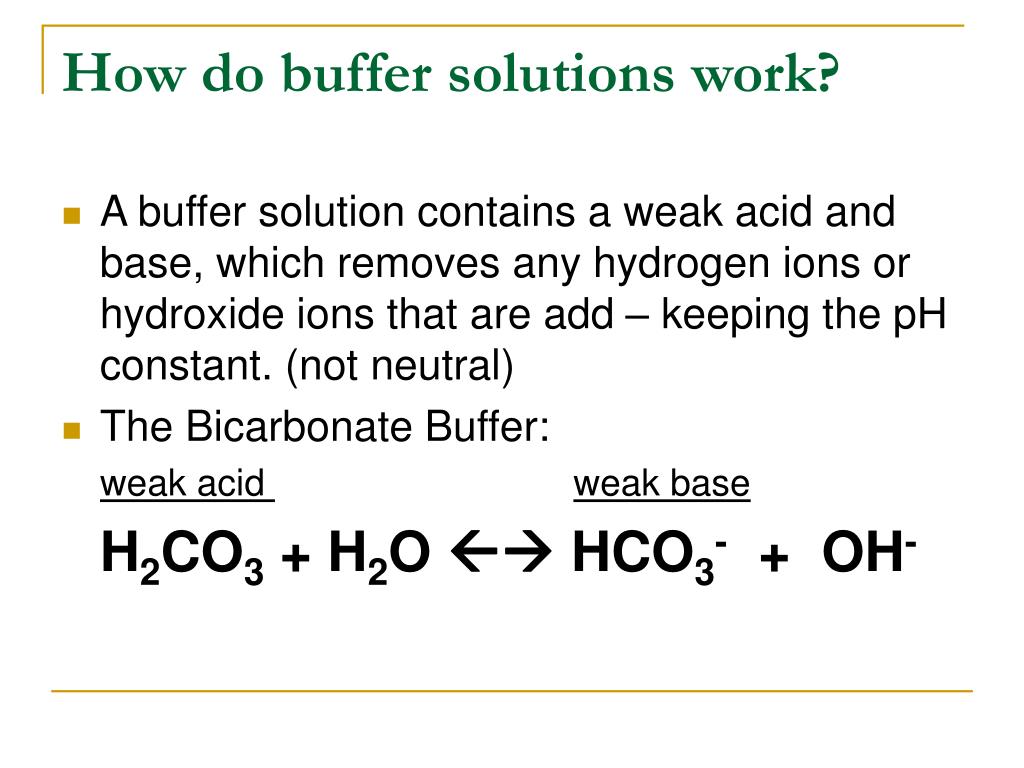
PPT Buffers PowerPoint Presentation, free download ID5687114
Related Post: