Calcium Reacts With Fluorine To Form
Calcium Reacts With Fluorine To Form - Ca + f_2 → caf_2. Web as a result of receiving the two electrons, calcium will have a negative charge. Ca + f2 → caf2. Web the evidence of the uptake of fluorine by calcium carbonate is clear, and we have identified the likely mechanism of uptake. Write the skeletal chemical equations of the following reactions. Web when calcium reacts with fluorine to produce calcium fluoride, calcium is oxidized and fluorine is reduced. Web web calcium reacts with fluorine to form what? You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web the reaction between calcium and fluorine can be represented by the following equation: The two fluorides become positive from losing an electron. Calcium reacts with fluorine gas to produce. (5 items x 2 points) 1. Web the evidence of the uptake of fluorine by calcium carbonate is clear, and we have identified the likely mechanism of uptake. Web this problem has been solved! Web as a result of receiving the two electrons, calcium will have a negative charge. Web this problem has been solved! So, atoms of fluorine gain. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Calcium reacts with fluorine gas to produce. Calcium reacts with fluorine gas to. The earlier observations that aragonite is. Web so in the given process of calcium fluoride, the one electron from the valence shell of calcium will be released making it as ions and this is termed as oxidation process. Web calcium (ca) reacts with fluorine gas (f2) to produce calcium fluoride (caf2). You'll get a detailed solution from a subject matter. The positive and negative charge. Web the reaction between calcium and fluorine can be represented by the following equation: You'll get a detailed solution from a subject matter expert that helps you learn core concepts. The two fluorides become positive from losing an electron. (5 items x 2 points) 1. Calcium reacts with fluorine gas to produce calcium fluoride. The two fluorides become positive from losing an electron. Ca + f2 → caf2. (5 items x 2 points) 1. Web calcium (ca) reacts with fluorine gas (f2) to produce calcium fluoride (caf2). Calcium reacts with fluorine gas to produce calcium fluoride. The earlier observations that aragonite is. Calcium reacts with fluorine gas to. The positive and negative charge. Web this problem has been solved! So, atoms of fluorine gain. Ca + f2 → caf2. From the question, we are to explain how oxidation. In this reaction, one atom of calcium combines with one molecule of. Web you'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web as a result of receiving the two electrons, calcium will have a negative charge. Web calcium (ca) reacts with fluorine gas (f2) to produce calcium fluoride (caf2). Write the skeletal chemical equations of the following reactions. The balanced chemical equation for the given reaction is ca + f2 → caf2. What volume of fluorine gas (in ml) is required. Web calcium reacts with fluorine to form what? Calcium reacts with fluorine gas to produce calcium fluoride. Web chemistry questions and answers. Web the reaction between calcium and fluorine can be represented by the following equation: The balanced chemical equation for the given reaction is ca + f2 → caf2. Web when calcium reacts with fluorine to produce calcium fluoride, calcium is oxidized and fluorine is reduced. Write the skeletal chemical equations of the following reactions. Web calcium fluoride (caf2) is an insoluble ionic compound composed of ca 2+ and f − ions. Web web calcium reacts with fluorine to form what? What volume of fluorine gas (in ml) is. Web web calcium reacts with fluorine to form what? The balanced chemical equation for the given reaction is ca + f2 → caf2. Ca + f_2 → caf_2. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. The two fluorides become positive from losing an electron. Fluorine gas reacts with solid calcium bromide to form calcium fluoride and liquid bromine. Web when calcium reacts with fluorine to produce calcium fluoride, calcium is oxidized and fluorine is reduced. Web calcium (ca) reacts with fluorine gas (f2) to produce calcium fluoride (caf2). From the question, we are to explain how oxidation. Write the skeletal chemical equations of the following reactions. Calcium reacts with fluorine gas to produce. Web chemistry questions and answers. Calcium reacts with fluorine gas to produce calcium fluoride. So, atoms of fluorine gain. Web so in the given process of calcium fluoride, the one electron from the valence shell of calcium will be released making it as ions and this is termed as oxidation process. Web you'll get a detailed solution from a subject matter expert that helps you learn core concepts. Calcium reacts with fluorine gas to. Web the evidence of the uptake of fluorine by calcium carbonate is clear, and we have identified the likely mechanism of uptake. Web this problem has been solved! Web first of all, we're told that calcium reacts of flooring to form calcium fluoride and each of the reaction in the reaction, each atom of one of the elements loses two electrons.science chemistry precipitation reaction calcium fluoride Fundamental
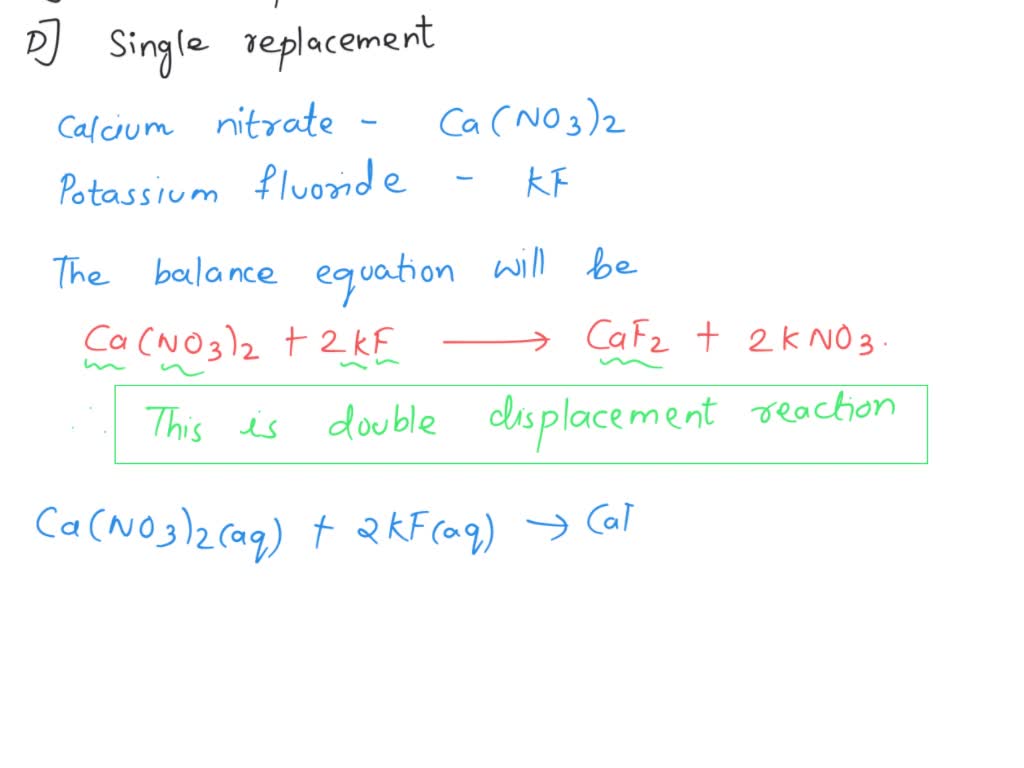
SOLVED 'Calcium nitrate and potassium fluoride solutions react to form
Calcium Fluoride And Sulfuric Acid Make Calcium Sulfate And Hydrogen
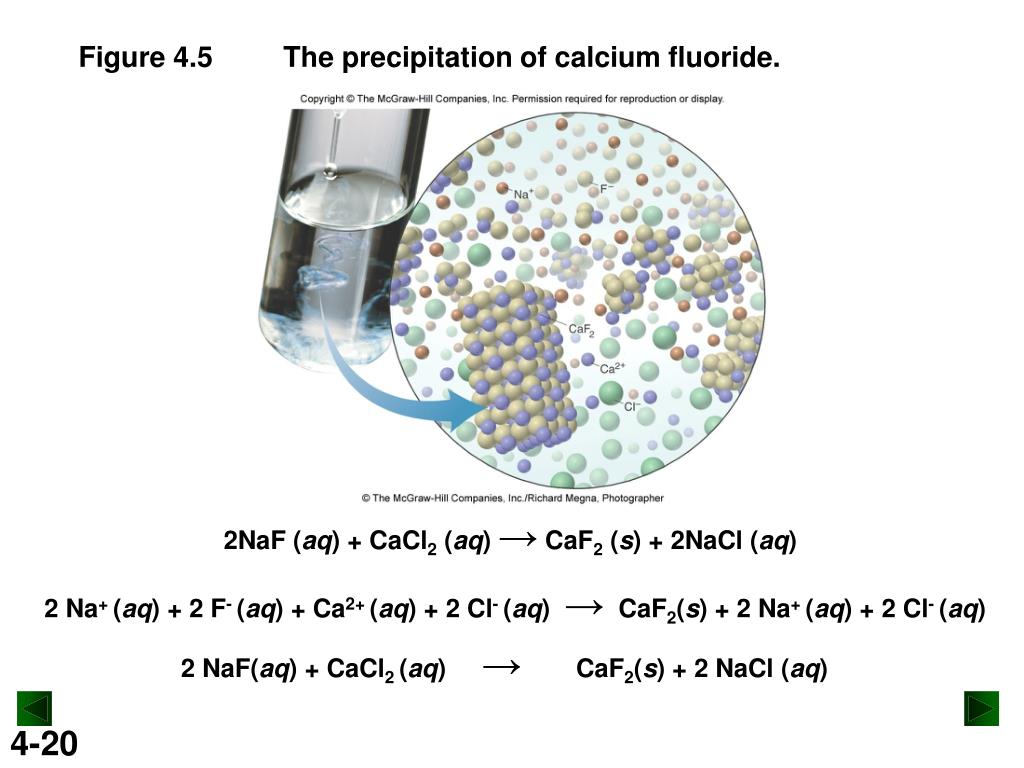
science chemistry precipitation reaction calcium fluoride Fundamental
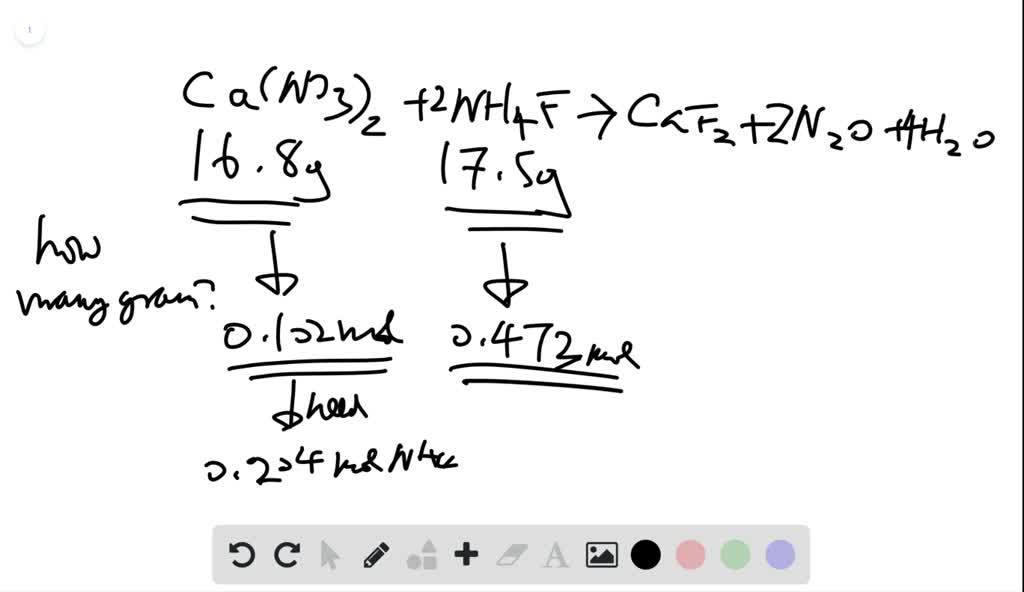
SOLVEDCalcium nitrate and ammonium fluoride react to form calcium
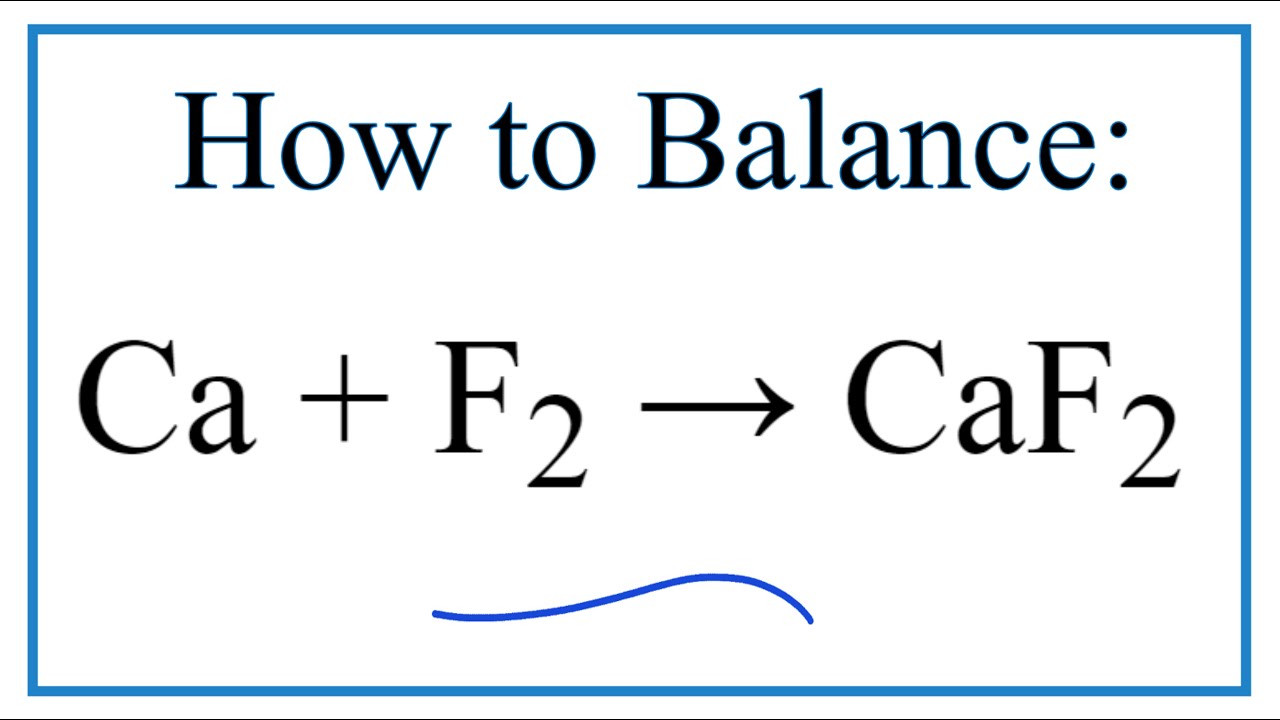
How to Balance Ca + F2 = CaF2 (Calcium + Fluorine gas) YouTube
PPT Chapter 4 PowerPoint Presentation, free download ID2948816
SOLVEDIf atoms of the metal calcium were to react with molecules of
SOLVEDCalcium reacts with fluorine to form the compound CaF2. In the
science chemistry precipitation reaction calcium fluoride Fundamental
Related Post: