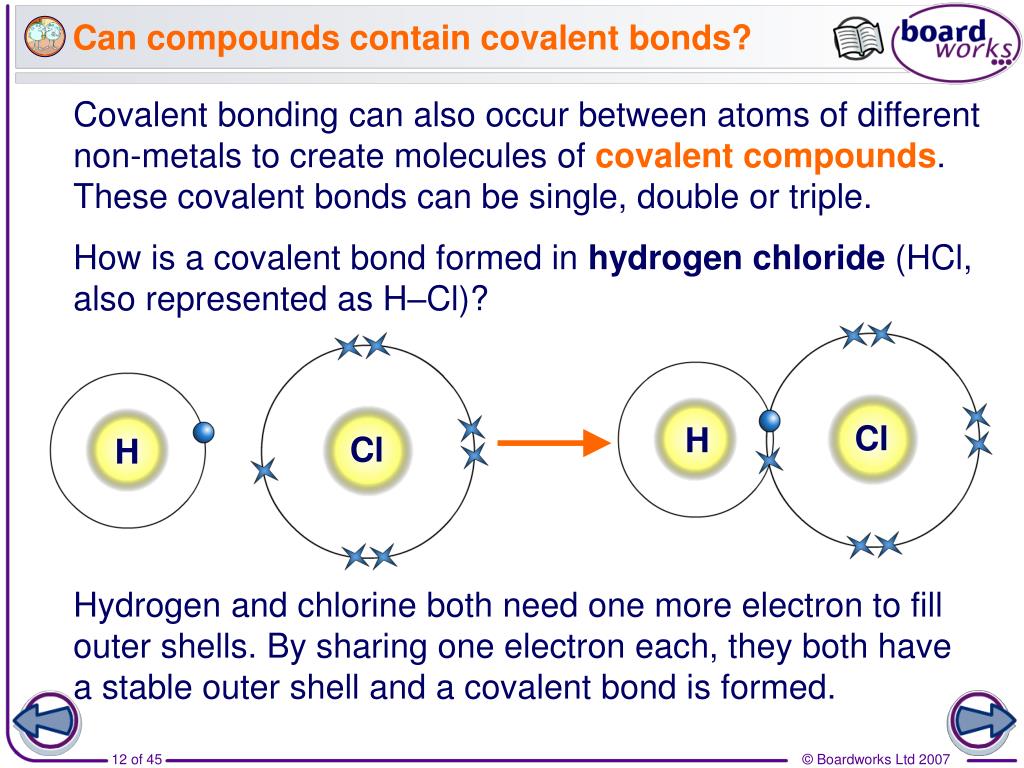
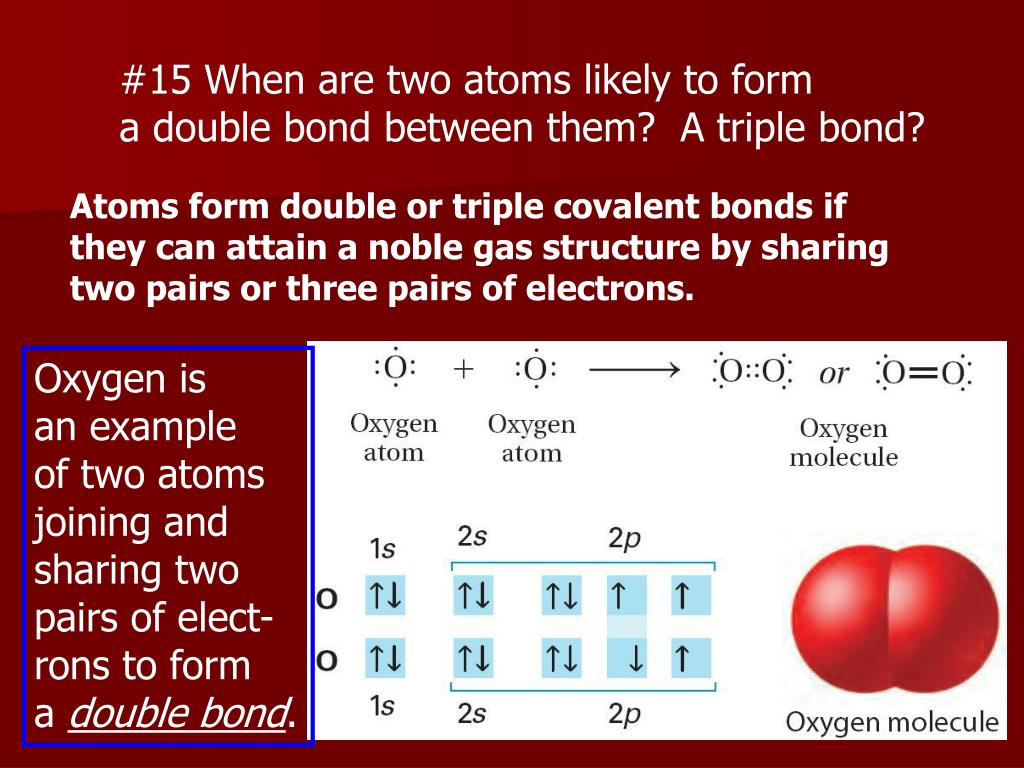
Which Two Atoms Would Typically Form A Covalent Bond
Which Two Atoms Would Typically Form A Covalent Bond - Group 5a form 3 bonds; Group 6a form 2 bonds; Web a nonpolar covalent bond is created when atoms share their electrons equally. A metal atom and a nonmetal atom o d. Boron commonly makes only three covalent bonds, resulting in only six valence electrons around the b b atom. Group 5a form 3 bonds; The h atom has a complete valence shell. This chapter will focus on the properties of covalent compounds. For example, the hydrogen molecule, h 2, contains a covalent bond between its two hydrogen atoms. A neutral hydrogen atom, shown left, contains one electron. In polar covalent bonds, the electrons are shared unequally, as one atom exerts a stronger force of attraction on the electrons than the other. Web typically, the atoms of group 4a form 4 covalent bonds; Nonmetal atoms frequently form covalent bonds with other nonmetal atoms. Web formation of covalent bonds. Web covalent bonds are formed between two atoms when both. Covalent bond is defined as a type of bond which is formed by the mutual sharing of electrons to form electron pairs between the two atoms.these electron pairs are called as bonding pairs or shared pair of electrons. However, the o atom has only seven electrons around it, which is not a. And group 7a form one bond. Each hydrogen. Web the two atoms which have low electronegativity difference that is they are non metals can form a covalent bond. For example, the hydrogen molecule, h 2, contains a covalent bond between its two hydrogen atoms. The closer the values of their electron affinity, the stronger the attraction. Is determined by the distance at which the lowest potential energy is. Web formation of covalent bonds. Group 6a form 2 bonds; And group 7a form one bond. In pure covalent bonds, the electrons are shared equally. And group 7a form one bond. 4.1 introduction to covalent molecules and compounds. Two atoms that are both nonmetals o b. Two ions of equal and opposite charge submit Web a covalent bond forming h 2 (right) where two hydrogen atoms share the two electrons. These electron pairs are known as shared pairs or bonding pairs. Web covalent bonds are formed between two atoms when both have similar tendencies to attract electrons to themselves (i.e., when both atoms have identical or fairly similar ionization energies and electron affinities). Thus, boron commonly forms three bonds, bh 3 , with a total of six electrons in the outermost shell. Group 5a form 3 bonds; The closer the values. An atom that shares one or more of its electrons. Web for example, two hydrogen atoms bond covalently to form an h 2 molecule; The closer the values of their electron affinity, the stronger the attraction. This chapter will focus on the properties of covalent compounds. 4.1 introduction to covalent molecules and compounds. Two atoms of very different electronegativities o c. The h atom has a complete valence shell. And group 7a form one bond. Web more than two atoms can participate in covalent bonding, although any given covalent bond will be between two atoms only. Web typically, the atoms of group 4a form 4 covalent bonds; Web there are two basic types of covalent bonds: An atom that shares one or more of its electrons. Group 5a form 3 bonds; The potential energy of two separate hydrogen atoms (right) decreases as they approach each other, and the single electrons on each atom are shared to form a covalent bond. Nonmetal atoms frequently form covalent bonds with. Nonmetal atoms frequently form covalent bonds with other nonmetal atoms. Web which two atoms would typically form a covalent bond? This usually occurs when two atoms have similar or the same electron affinity. An atom that shares one or more of its electrons. Consider h and o atoms: Figure \(\pageindex{1}\) illustrates why this bond is. A bond forms when the bonded atoms have a lower total energy than that of widely separated atoms. Boron commonly makes only three covalent bonds, resulting in only six valence electrons around the b b atom. The number of electrons required to obtain an octet determines the number of covalent bonds an atom can form. Group 6a form 2 bonds; Web covalent bonds form when electrons are shared between atoms and are attracted by the nuclei of both atoms. Web which two atoms would typically form a covalent bond? Is determined by the distance at which the lowest potential energy is achieved. A neutral hydrogen atom, shown left, contains one electron. Web covalent bonds are formed between two atoms when both have similar tendencies to attract electrons to themselves (i.e., when both atoms have identical or fairly similar ionization energies and electron affinities). The number of electrons required to obtain an octet determines the number of covalent bonds an. Web typically, the atoms of group 4a form 4 covalent bonds; Web a covalent bond is formed when two atoms share electron pairs. This occurs in gas molecules; Group 5a form 3 bonds; Group 6a form 2 bonds; And group 7a form one bond. Two ions of equal and opposite charge submit Web there are two basic types of covalent bonds: For example, the hydrogen molecule, h 2, contains a covalent bond between its two hydrogen atoms.Double Covalent Bond Definition and Examples
Ionic Bond Definition, Types, Properties & Examples
Polar vs. Nonpolar Bonds — Overview & Examples Expii
PPT Covalent Bonds PowerPoint Presentation, free download ID6647183
Chapter 5.6 Properties of Polar Covalent Bonds Chemistry LibreTexts
PPT Why do atoms form bonds? PowerPoint Presentation, free download
The top panel in this figure shows two hydrogen atoms sharing two
covalent bond Definition, Properties, Examples, & Facts Britannica
PPT Atomic structure and chemical bonding PowerPoint Presentation
PPT 13 p. 229 in the book What electron configurations do atoms
Related Post: