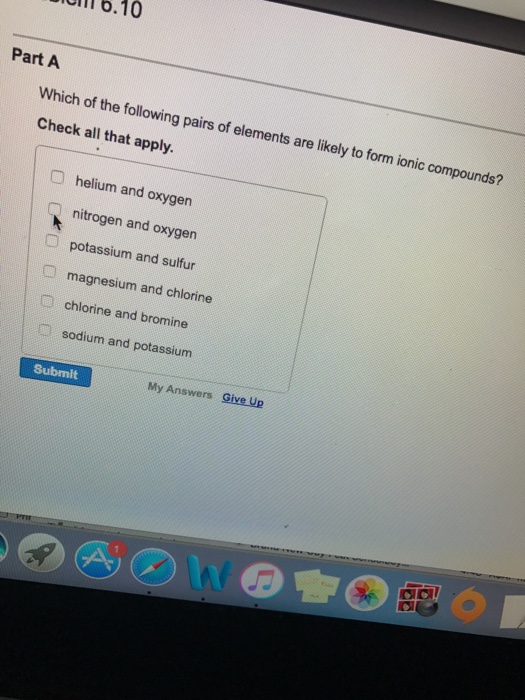
Which Pairs Of Elements Are Likely To Form Ionic Compounds
Which Pairs Of Elements Are Likely To Form Ionic Compounds - Web ionic compounds generally form from metals and nonmetals. Which of the following pairs of elements are likely to form ionic compounds? Web we would like to show you a description here but the site won’t allow us. Web chemistry chemistry questions and answers which of the following pairs of elements are likely to form ionic compounds? Which of the following pairs of elements are likely to form ionic compounds? The potential energy of two separate hydrogen atoms (right) decreases as. An ionic compound is formed from the combination of a metal iron, plus a nonmetal lion. An ionic compound is likely to be formed by which of the following pairs. Compounds between metal and nonmetal elements are usually ionic. Web which of the following pairs of elements are likely to form an ionic compound? Web the elements with high differences in electronegativity have been capable of forming ionic bonds. Compounds that do not contain ions, but instead consist of atoms bonded tightly together in molecules (uncharged groups of atoms that behave as a single unit), are called covalent compounds. Web chemistry chemistry questions and answers which pair of elements is most likely to form. Web which of the following pairs are likely to form an ionic compound. Is determined by the distance at which the lowest potential energy is achieved. Web chemistry chemistry questions and answers which of the following pairs of elements are likely to form ionic compounds? An ionic compound is formed from the combination of metal iron and a nonmetal lion.. Web we would like to show you a description here but the site won’t allow us. Magnesium and fluorine the pairs of elements that is most likely to form an ionic compound is magnesium and. Web the elements with high differences in electronegativity have been capable of forming ionic bonds. The following pairs of elements: Web which of the following. An ionic compound is formed from the combination of metal iron and a nonmetal lion. Web chemistry chemistry questions and answers which of the following pairs of elements are likely to form ionic compounds? Which of the following pairs of elements are likely to form ionic compounds? An ionic compound is likely to be formed by which of the following. Which of the following pairs of elements are likely to form ionic compounds? An ionic compound is formed from the combination of a metal iron, plus a nonmetal lion. Is determined by the distance at which the lowest potential energy is achieved. Web the set of pairs of elements whose atoms will combine to form a compound called ionic compound. Which of the following pairs of elements are likely to form an ionic. Which of the following pairs of elements are likely to form ionic compounds? Web which of the following pairs are likely to form an ionic compound. For example, cabr2 cabr 2 contains a metallic element (calcium, a group 2 [or. Web the correct answer to this question. Web which of the following pairs are likely to form an ionic compound. S and f c and o si and cl n and o cu. Which of the following pairs of elements are likely to form an ionic. Compounds between metal and nonmetal elements are usually ionic. Web chemistry chemistry questions and answers which of the following pairs of. The following pairs of elements: An ionic compound is formed from the combination of metal iron and a nonmetal lion. For example, cabr2 cabr 2 contains a metallic element (calcium, a group 2 [or. Which of the following pairs of elements are likely to form an ionic. Which of the following pairs of elements are likely to form ionic compounds? Web which of the following pairs are likely to form an ionic compound. An ionic compound is formed from the combination of a metal iron, plus a nonmetal lion. Web the set of pairs of elements whose atoms will combine to form a compound called ionic compound are:. Compounds between metal and nonmetal elements are usually ionic. Web ionic compounds. S and f c and o si and cl n and o cu. For example, cabr2 cabr 2 contains a metallic element (calcium, a group 2 [or. Web which of the following pairs of elements are likely to form an ionic compound? An ionic compound is formed from the combination of metal iron and a nonmetal lion. An ionic compound. Web the set of pairs of elements whose atoms will combine to form a compound called ionic compound are:. Web in the given pairs, magnesium and chlorine (b) and nitrogen and oxygen (f) are most likely to form ionic compounds because of their large electronegativity difference. Web chemistry chemistry questions and answers which of the following pairs of elements are likely to form ionic compounds? Which of the following pairs of elements are likely to form ionic compounds? An ionic compound is likely to be formed by which of the following pairs. Compounds between metal and nonmetal elements are usually ionic. Web the correct answer to this question is letter d. An ionic compound is formed from the combination of a metal iron, plus a nonmetal lion. For example, cabr2 cabr 2 contains a metallic element (calcium, a group 2 [or. The following pairs of elements: Magnesium and fluorine the pairs of elements that is most likely to form an ionic compound is magnesium and. Web ionic compounds generally form from metals and nonmetals. Web which of the following pairs of elements are likely to form an ionic compound? S and f c and o si and cl n and o cu. Which of the following pairs of elements are likely to form ionic compounds? Web we would like to show you a description here but the site won’t allow us. Web which of the following pairs are likely to form an ionic compound. Compounds that do not contain ions, but instead consist of atoms bonded tightly together in molecules (uncharged groups of atoms that behave as a single unit), are called covalent compounds. Which of the following pairs of elements are likely to form ionic compounds? The potential energy of two separate hydrogen atoms (right) decreases as.Ionic Compounds
What are Ionic Compounds and how they are formed?
Which of the following pairs of elements is most likely to form an
Ionic Bonding Presentation Chemistry
Ionic Bond Definition, Types, Properties & Examples
The top panel in this figure shows two hydrogen atoms sharing two
Ionic Bond Definition, Types, Properties & Examples
Solved Which of the following pairs of elements are likely
Examples of Ionic Compoiunds YouTube
Naming Simple Ionic Compounds Pathways to Chemistry
Related Post:
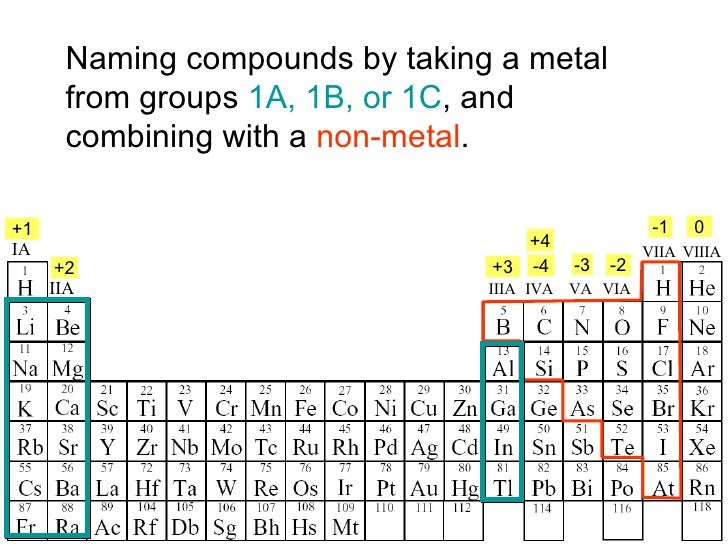


.PNG)