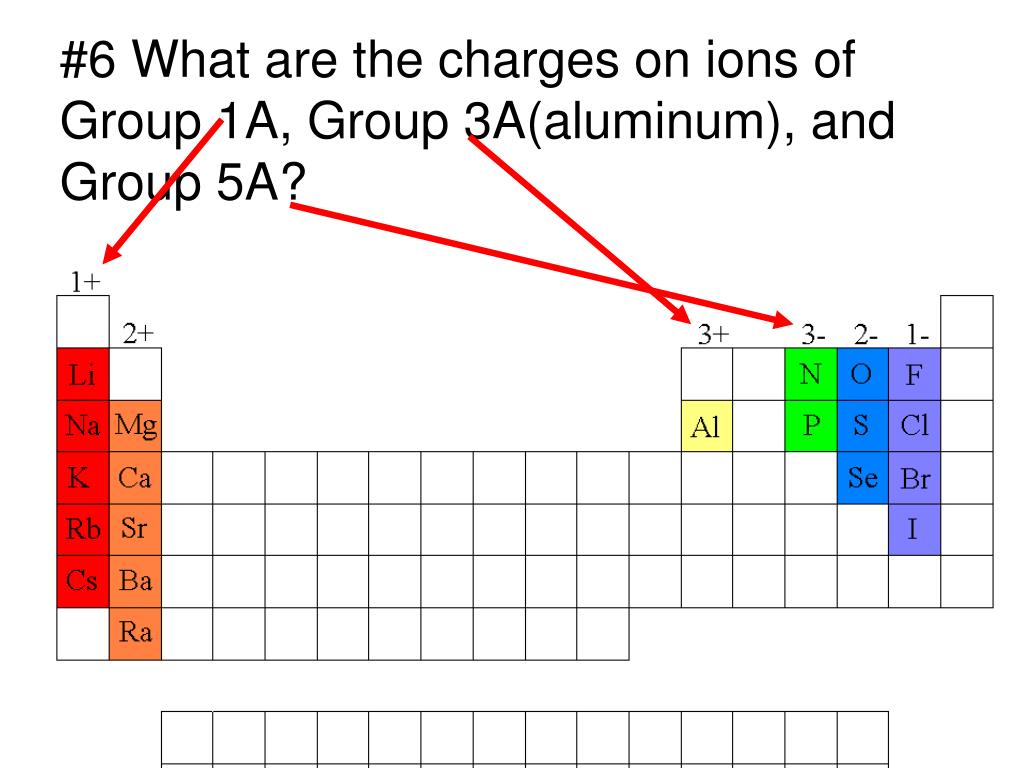
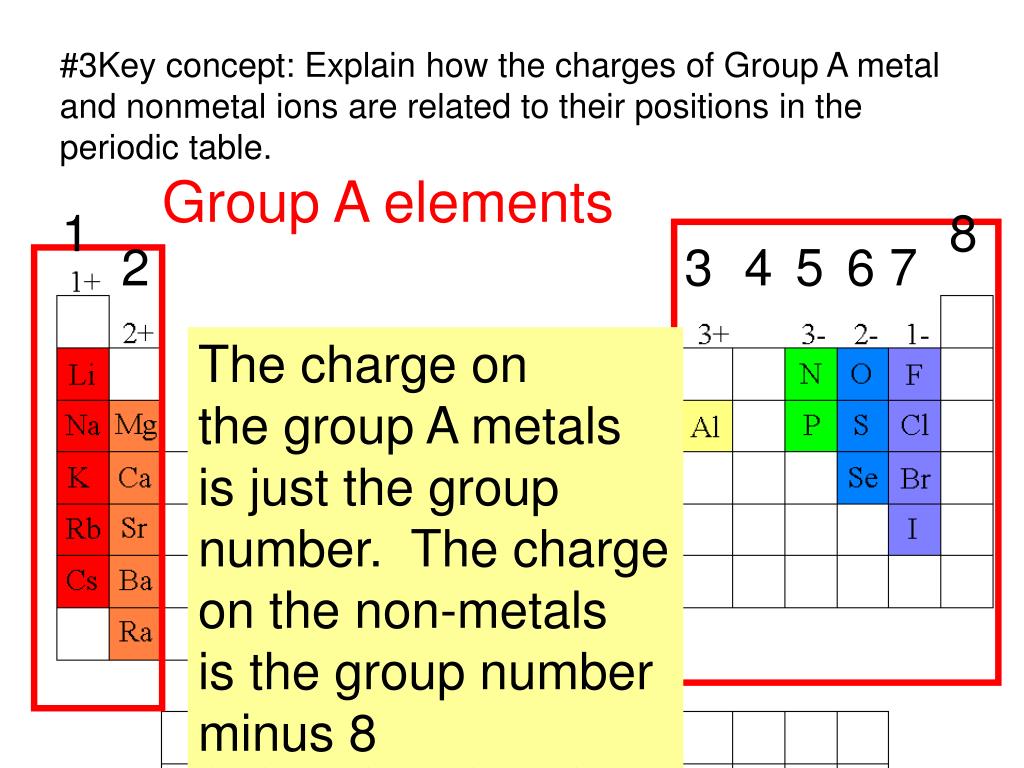
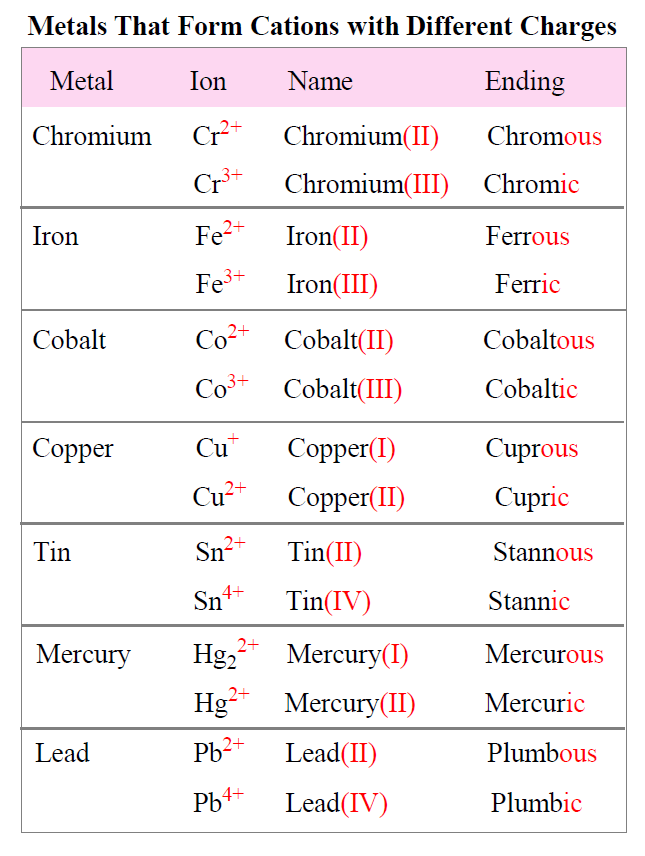
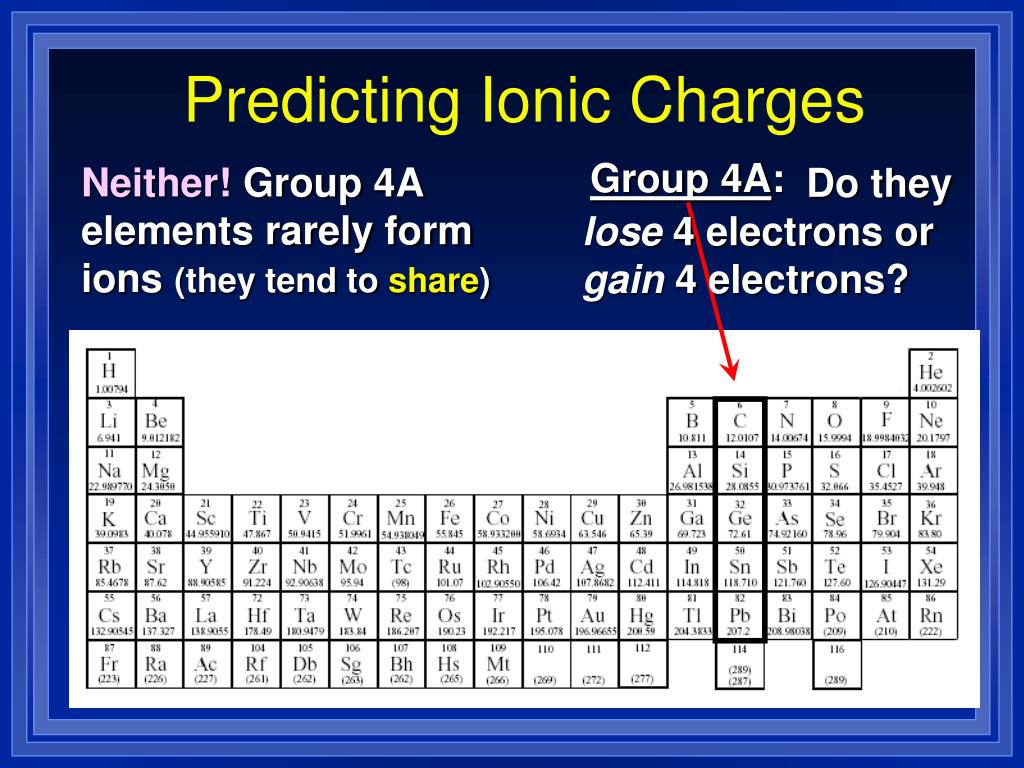
Which Metal Does Not Form Cations Of Differing Charges
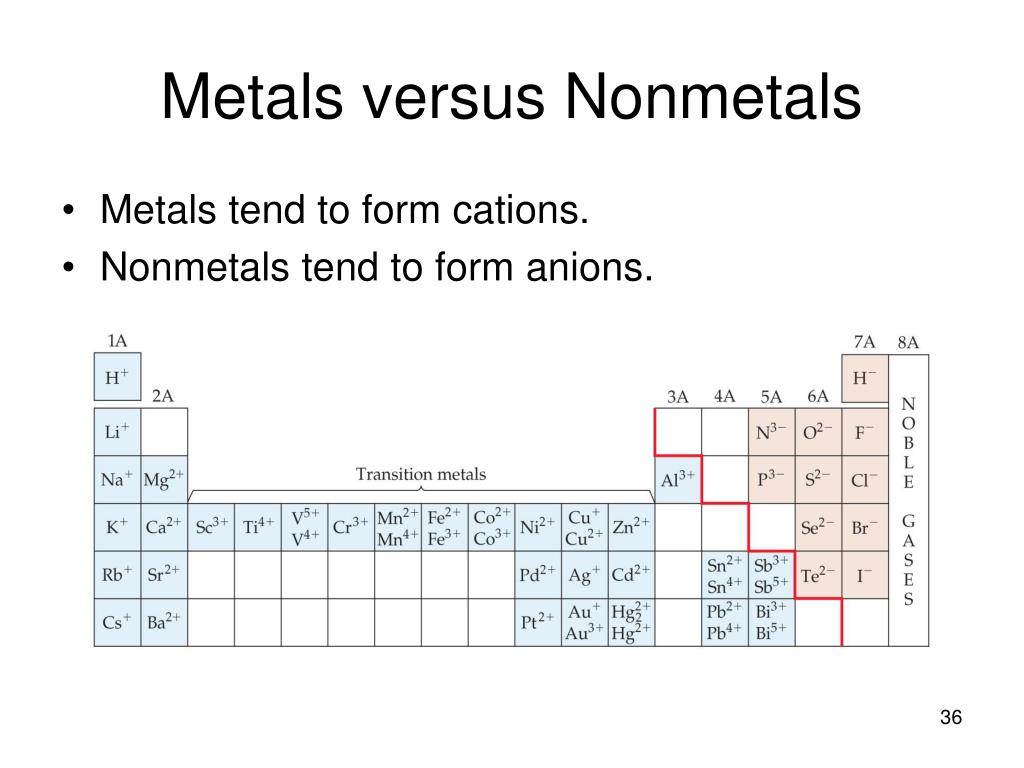
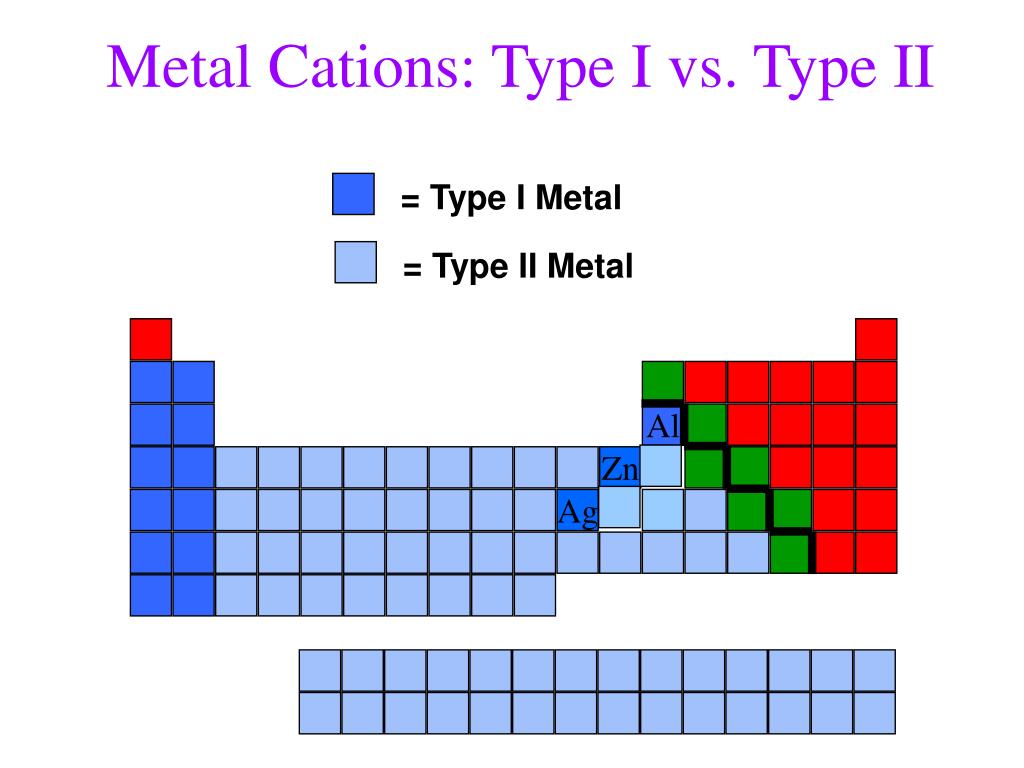
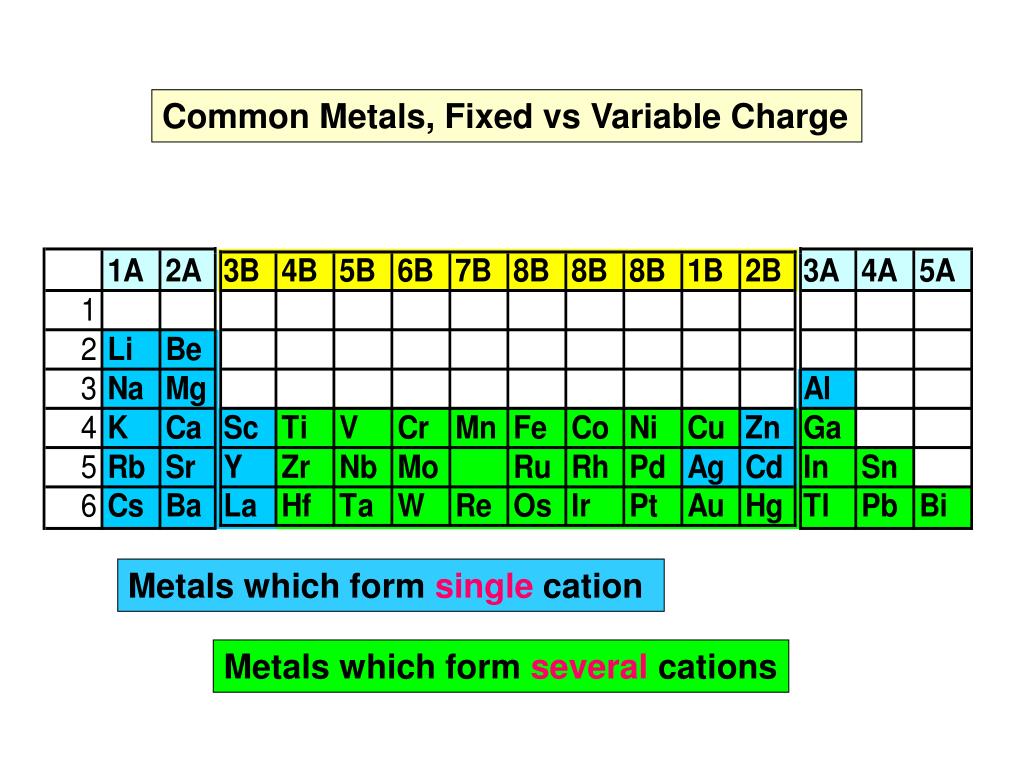
Which Metal Does Not Form Cations Of Differing Charges - The transition metals refers to the group of metals which one can find in the middle of a periodic table. A) the law of multiple proportions. Which one of the following compounds is copper(i) chloride? In addition, some metals may produce the same color and thus can not differentiate all metals. For metals the symbol is mex+. Web which metal does not form cations of differing charges? Metal atoms tend to lose electrons. Web you'll get a detailed solution from a subject matter expert that helps you learn core concepts. Which metal does not form cations of differing charges? The type of ions that metals form are called positively. Chronium (cr) , iron (fe) , cobalt (co) , copper (cu) , tin (sn) , mercury (hg). Web aluminum reacts with a certain nonmetallic element to form a compound with the general formula alx. Web of the choices below, which one is not an ionic compound? A molecule of water contains hydrogen and oxygen in a 1:8 ratio by mass.. Which metal does not form cations of differing charges? Pbcl2 (aq) + k2cro4 (aq) →. For metals the symbol is mex+. A) pc15 b) moc16 c) rbci d) pbcl2 e) naci 16. Which metal has the same charge in all of it's compounds? Which one of the following compounds is copper(i) chloride? The transition metals refers to the group of metals which one can find. Web you'll get a detailed solution from a subject matter expert that helps you learn core concepts. For metals the symbol is mex+. Correct option is b) na. Correct option is b) na. Many metals, on the other. Web cation is a positive ion, not an element; The type of ions that metals form are called positively. Web the metal that does not form cations of differing charges is sodium (na). Web the metal that does not form cations of differing charges is sodium (na). Sodium, on the other hand, is an alkali metal belonging to. Which metal has the same charge in all of it's compounds? What type ions do metals form? Web of the choices below, which one is not an ionic compound? Correct option is b) na. Web of the choices below, which one is not an ionic compound? Transition metals form cations with different. The transition metals refers to the group of metals which one can find. Web these metals can form cations with diverse charges depending on the specific compound and reaction conditions. Which metal has the same charge in all of it's compounds? The team is made of iron as n 10 and s. Terms in this set (24) metals that form cations with different charges. Web which metal does not form cations of differing charges? Transition metals form cations with different. Web of the choices below, which one is not an ionic compound? Which one of the following compounds is copper(i) chloride? Web these metals can form cations with diverse charges depending on the specific compound and reaction conditions. The transition metals refers to the group of metals which one can find. This is a statement of ________. Terms in this set (24) metals that form cations with different charges. Which one of the following compounds is copper(i) chloride? Metal atoms tend to lose electrons. The transition metals refers to the group of metals which one can find in the middle of a periodic table. Many metals, on the other. Which metal does not form cations of differing charges? A) pc15 b) moc16 c) rbci d) pbcl2 e) naci 16. Many metals, on the other. Web these metals can form cations with diverse charges depending on the specific compound and reaction conditions. Element x is a diatomic gas at room temperature. Web which element forms an ion with the same charge as the sulfite ion? Web cation is a positive ion, not an element; Web you'll get a detailed solution from a subject matter expert that helps you learn core concepts. The transition metals refers to the group of metals which one can find. Sodium, on the other hand, is an alkali metal belonging to. The correct answer will be na as the na have only 1. Metal atoms tend to lose electrons. The type of ions that metals form are called positively. Transition metals form cations with different. Pbcl2 (aq) + k2cro4 (aq) →. Which metal does not form cations of differing charges?. Element x is a diatomic gas at room temperature. When a fluorine atom forms the florid ion, it has the same charge as the _____ ion. Web aluminum reacts with a certain nonmetallic element to form a compound with the general formula alx. Web the metal that does not form cations of differing charges is sodium (na). Web these metals can form cations with diverse charges depending on the specific compound and reaction conditions. A) na b) cu c) co d) fe e) sn answer: This is a statement of ________. Web the metal that does not form cations of differing charges is sodium (na). We have some options here.Element Charges Chart How to Know the Charge of an Atom Science
PPT Chapter 9 Chemical Names and Formulas Section 9.1 Naming Ions
PPT 1 Name the ions formed by these elements and classify them as
PPT 1 Name the ions formed by these elements and classify them as
Writing Chemical Formulas For Ionic Compounds Chemistry Steps
PPT Chapter 9 “Chemical Names and Formulas” PowerPoint Presentation
PPT Orbital Diagrams and Electron Configuration PowerPoint
PPT Molecules and Compounds Nomenclature PowerPoint Presentation
PPT TOPIC II ELEMENTS AND COMPOUNDS PowerPoint Presentation, free
Cations and Anions Definitions, Examples, and Differences
Related Post: