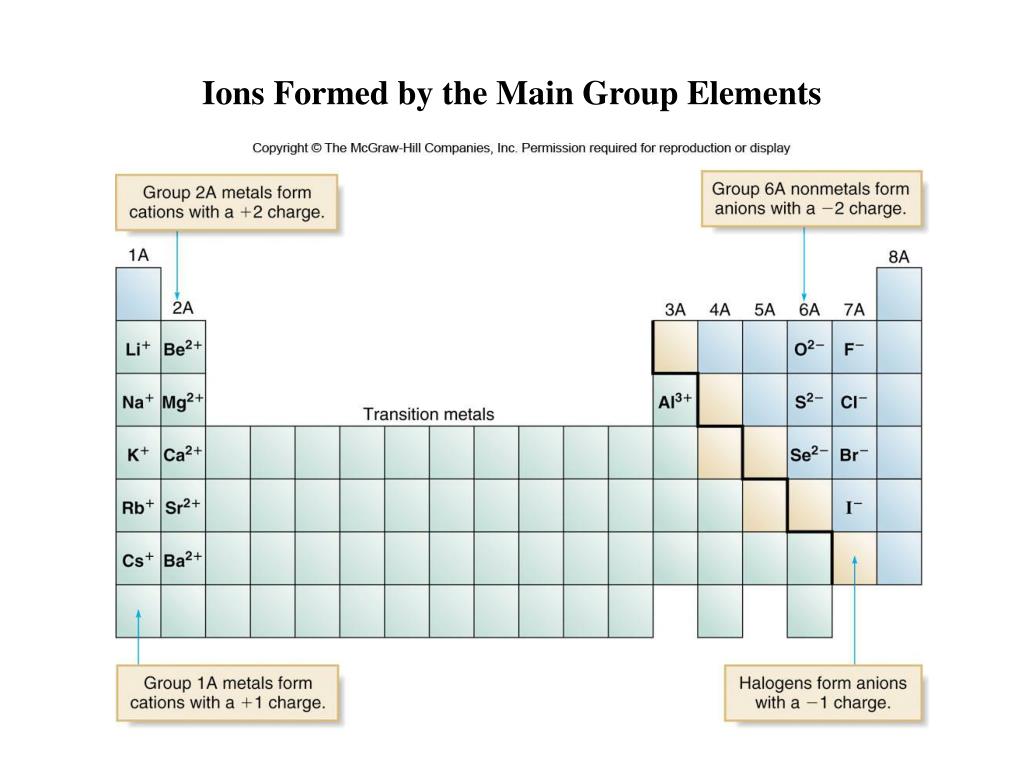
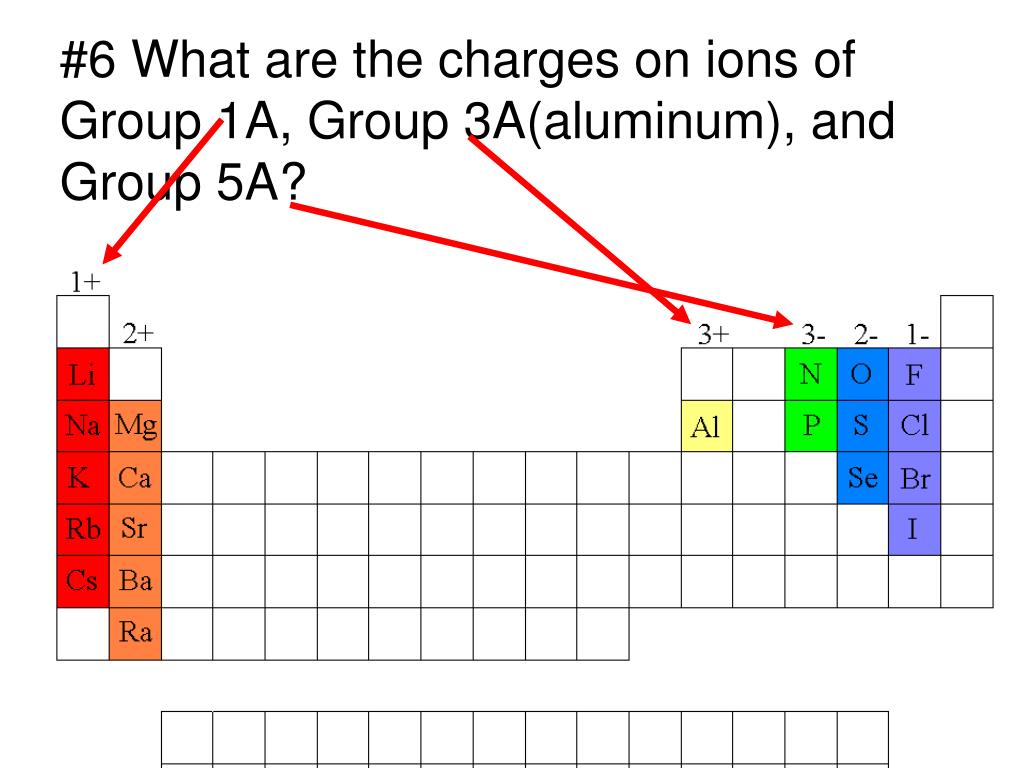
Which Group Tends To Form 1 Ions
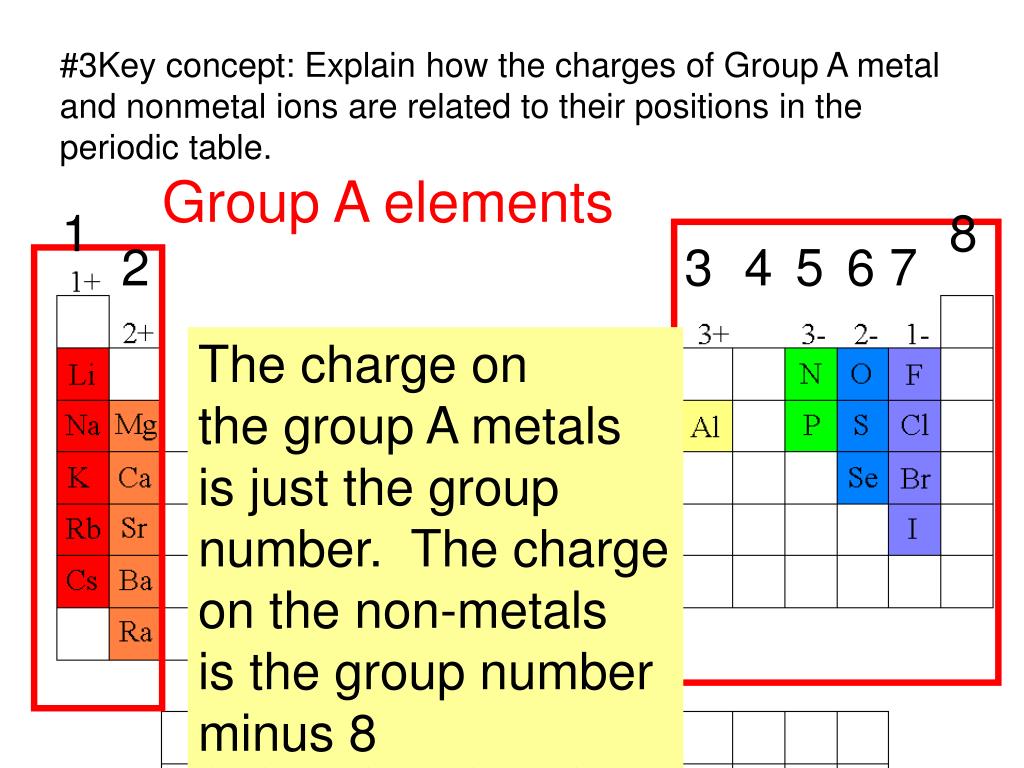
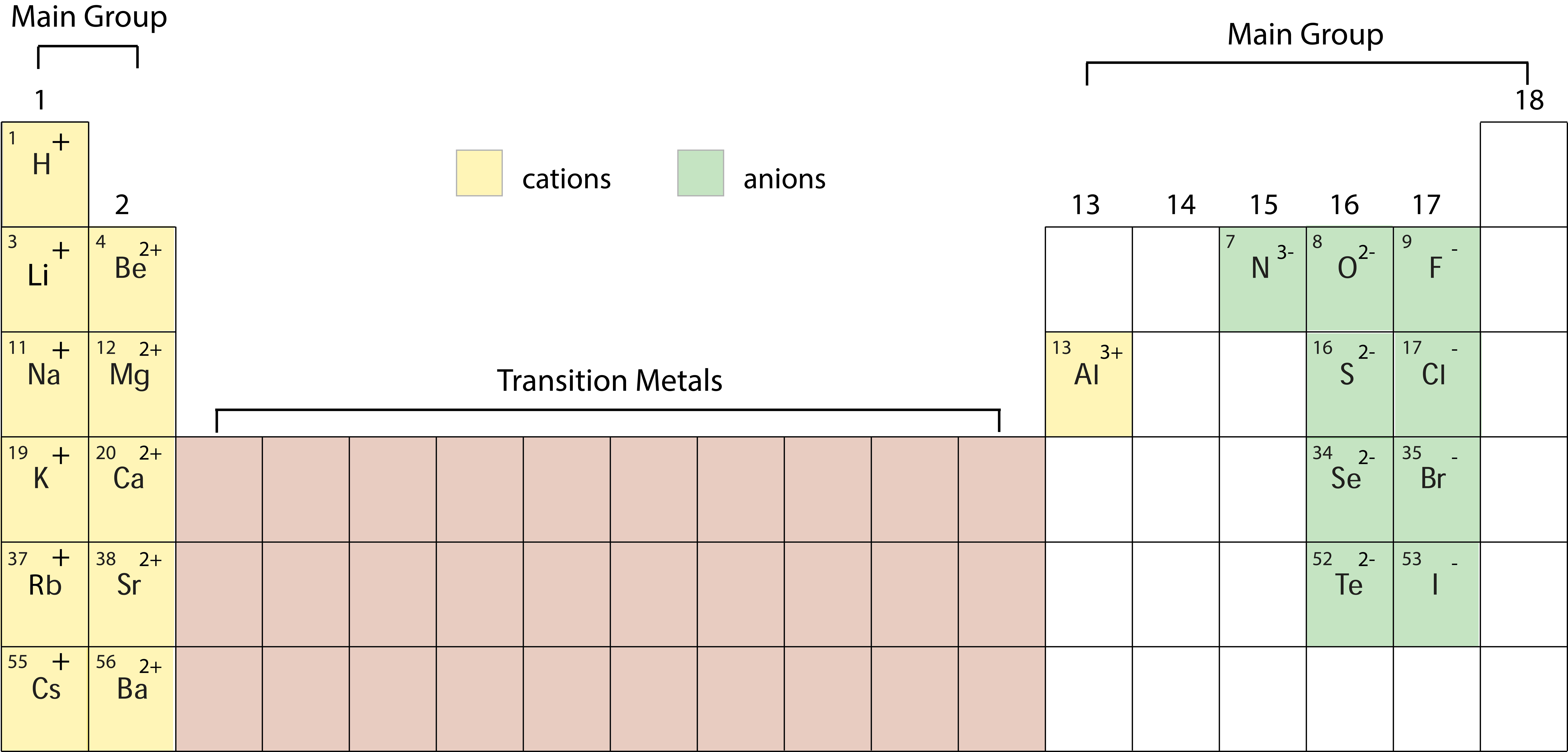
Which Group Tends To Form 1 Ions - Web which group tends to form 1+ ions? Because most metals form cations and most. Web the alkali metals have the lowest \(i_1\) values of the elements. Which group tends to form 2+ ions? This represents the relative ease with which the lone electron in the outer 's' orbital can be removed. The halogens, group 17, reach a full. Group 16 elements (two groups left) form 2− ions, and so on. Web for example, group 17 elements (one group left of the noble gases) form 1− ions; The alkali metals lose an electron to resemble the next lowest noble gas; Web group 1, the alkali metals. First, the cation is written before the anion. Web group 1, the alkali metals. Web for example, group 17 elements (one group left of the noble gases) form 1− ions; Web for example, group 17 elements (one group left of the noble gases) form 1− ions; Web for example, group 17 elements (one group left of the noble gases) form. Its because there's one valence electron in their outermost shell, they just lose that one electron and boom, they're stable. Web for example, group 17 elements (one group left of the noble gases) form 1− ions; Group 1 metals, the alkali metals, have the 1 valence electron, and thus form m + ions when oxidized. This trend can be used. Web for example, group 17 elements (one group left of the noble gases) form 1− ions; Group 1 metals, the alkali metals, have the 1 valence electron, and thus form m + ions when oxidized. An ionic compound is made up of charged particles, called ions. The alkali earth metals lose two electrons. So, all the alkali metals, group 1a,. Group 16 elements (two groups left) form 2− ions, and so on. The halogens, group 17, reach a full. Group 1 metals, the alkali metals, have the 1 valence electron, and thus form m + ions when oxidized. An ionic compound is made up of charged particles, called ions. Because most metals form cations and most. Web we would like to show you a description here but the site won’t allow us. Web for example, group 17 elements (one group left of the noble gases) form 1− ions; So, all the alkali metals, group 1a, tend to form a +1 charge. Group 16 elements (two groups left) form 2− ions, and so on. Web because the. The alkali metals lose an electron to resemble the next lowest noble gas; Which group tends to form 2+ ions? Web we would like to show you a description here but the site won’t allow us. Web the formula for an ionic compound follows several conventions. So, all the alkali metals, group 1a, tend to form a +1 charge. How many liters of nacl solution can i make if i want the. The general outer electronic configuration of alkali metals are ns1,np0 if they lose one. Ionization energy whats the opposite of electron affinity? The answer to this question is the alkali metals, which are located in group 1 of the periodic table. So, all the alkali metals, group. Web for example, group 17 elements (one group left of the noble gases) form 1− ions; First, the cation is written before the anion. Web dimensional analysis 1. This trend can be used as a. Web the formula for an ionic compound follows several conventions. The answer to this question is the alkali metals, which are located in group 1 of the periodic table. Group 1 metals, the alkali metals, have the 1 valence electron, and thus form m + ions when oxidized. Web the formula for an ionic compound follows several conventions. Web for example, group 17 elements (one group left of the noble. Web for example, group 17 elements (one group left of the noble gases) form 1− ions; Its because there's one valence electron in their outermost shell, they just lose that one electron and boom, they're stable. Web because the lanthanides and actinides formally belong to group 3, the most common ion formed by these elements is m 3 +, where. Web which group tends to form 1+ ions? Web for example, group 17 elements (one group left of the noble gases) form 1− ions; Group 16 elements (two groups left) form 2− ions, and so on. Group 16 elements (two groups left) form 2− ions, and so on. First, the cation is written before the anion. Web which group forms 1 ions? Group 2 metals, the alkaline earth metals, have 2 valence electrons, and thus form m 2+ ions. This trend can be used as a. Web dimensional analysis 1. The halogens, group 17, reach a full. The 1st group (alkali metals) tends to form +1 ions. Group 16 elements (two groups left) form 2− ions, and so on. An ionic compound is made up of charged particles, called ions. Noble gases which group tends to not form ions or react? Web the formula for an ionic compound follows several conventions. This represents the relative ease with which the lone electron in the outer 's' orbital can be removed. I have 470 milligrams of table salt, which is the chemical compound nacl. It has a giant lattice structure with strong electrostatic forces of attraction. The alkali earth metals lose two electrons. Web we would like to show you a description here but the site won’t allow us.Ionic Compound Nomenclature Presentation Chemistry
Ions
PPT Atoms, Ions, and Molecules Matter Starts Here PowerPoint
PPT Chapter 6 Chemical Nomenclature PowerPoint Presentation, free
PPT 1 Name the ions formed by these elements and classify them as
PreChemistry
Ionic Compound Nomenclature Presentation Chemistry
PPT Ionic Compounds Introduction to Bonding PowerPoint Presentation
Chem Types of Chemical Equations Scientific Tutor
PPT 1 Name the ions formed by these elements and classify them as
Related Post:
.PNG)

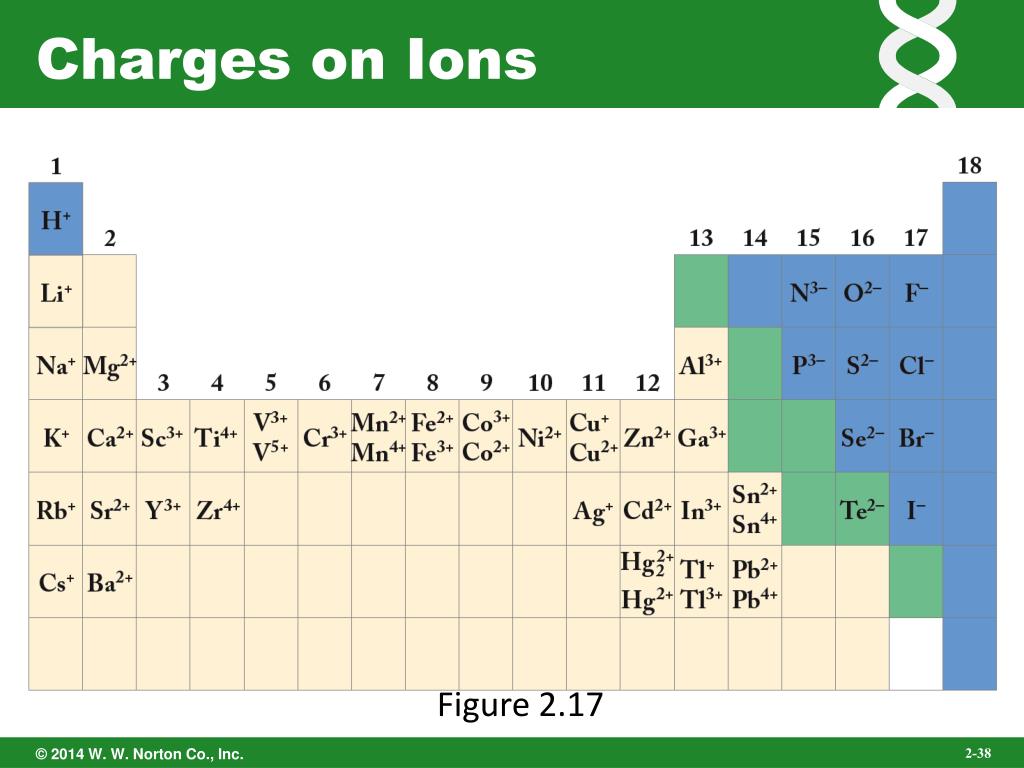
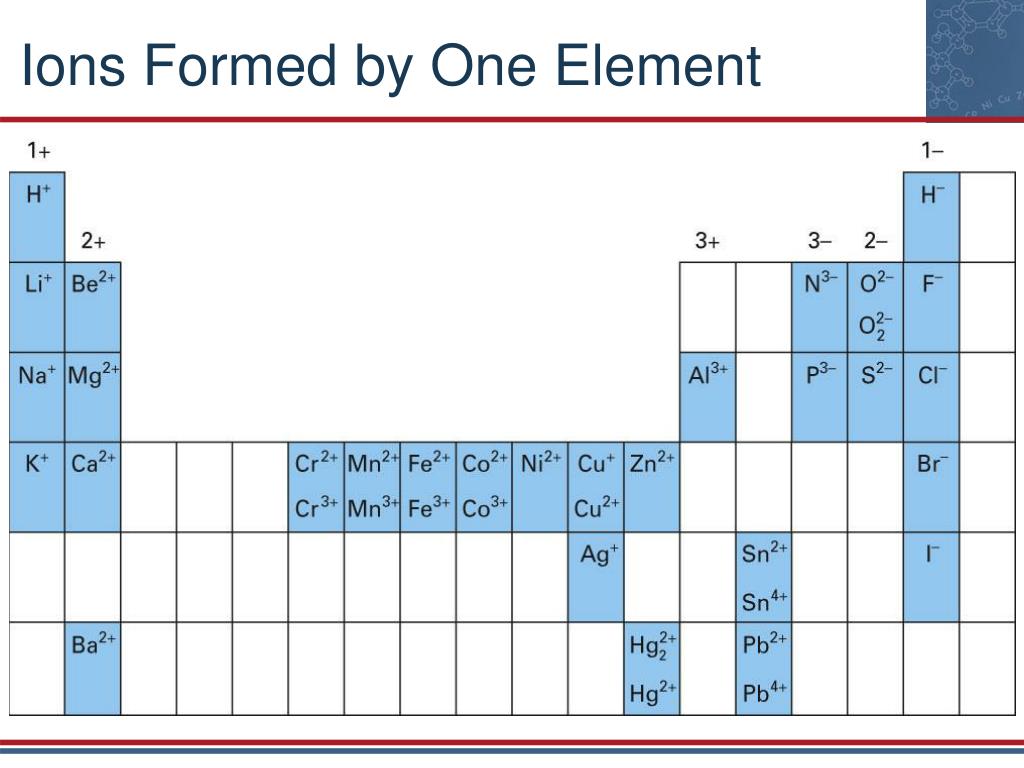
.PNG)