Which Atom Is Most Likely To Form A 1 Ion
Which Atom Is Most Likely To Form A 1 Ion - Atoms of group 16 gain two electrons and form ions with a 2− charge, and so on. Web ions are stable atoms of elements formed when original atoms loose or gain electrons to form stable configuration. Web which one of the following elements is most likely to form a 1+ ion in ionic compound ? Web note the usefulness of the periodic table in predicting likely ion formation and charge (figure 2.29). Web atoms of group 17 gain one electron and form anions with a 1− charge; Which atom is most likely to accept electrons to form an ionic bond? Iodine and potassium form an ionic bond. The most likely element to form a 1+ ion in ionic compound is. For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it with. A) ar b) br c) mg d) p e) k. Web atoms of group 17 gain one electron and form anions with a 1− charge; Web iodine becomes an ion with a negative 1 charge. (review the periodic table to view the elements) a. Iodine and potassium form an ionic bond. Which two elements will most likely form an ionic bond? Web what atom is most likely to form a ion? Ad browse & discover thousands of science book titles, for less. Ionic compounds form because ___________. Web about transcript when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. The most likely element to form a 1+ ion in ionic compound. Which two elements will most likely form an ionic bond? Web up to $3 cash back start filling in the gaps now. For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it with. A) ar b) br c) mg d) p e) k. Iodine and potassium form an ionic bond. The relative charge of a proton is +1. Iodine and potassium form an ionic bond. Anions are negatively charged ions formed when. Web the atomic number is equal to the number of neutrons in the nucleus of an atom (correct: Get the answers you need, now! Web atoms of group 17 gain one electron and form anions with a 1− charge; Atoms of group 16 gain two electrons and form ions with a 2− charge, and so on. We also find many polyatomic ions. You'll get a detailed solution from a subject matter expert that helps you. The relative charge of a proton is +1. Get the answers you need, now! Web up to $3 cash back start filling in the gaps now. (a) ar (b) br (c) mg (d) p (e) k. Web which atom is most likely to accept electrons to form an ionic bond? Which two elements will most likely form an ionic bond? A) ar b) br c) mg d) p e) k ions: (a) ar (b) br (c) mg (d) p (e) k. For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it with. Web about transcript when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve. Web the atomic number is equal to the number of neutrons in the nucleus of an atom (correct: A) ar b) br c) mg d) p e) k ions: Web atoms of group 17 gain one electron and form anions with a 1− charge; Web ions are stable atoms of elements formed when original atoms loose or gain electrons to. The most likely element to form a 1+ ion in ionic compound is. Web the atomic number is equal to the number of neutrons in the nucleus of an atom (correct: Get the answers you need, now! Ad browse & discover thousands of science book titles, for less. A) ar b) br c) mg d) p e) k. Which atom is most likely to accept electrons to form an ionic bond? For example, fluorine has seven valence. Web up to $3 cash back start filling in the gaps now. Ad browse & discover thousands of science book titles, for less. Get the answers you need, now! Anions are negatively charged ions formed when. Get the answers you need, now! Web ions are stable atoms of elements formed when original atoms loose or gain electrons to form stable configuration. Web atoms of group 17 gain one electron and form anions with a 1− charge; Atoms of group 16 gain two electrons and form ions with a 2− charge, and so on. Web about transcript when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. Iodine and potassium form an ionic bond. Web which two elements will most likely form an ionic bond? Web the ions are positive, because they have more protons close proton subatomic particle with a positive charge and a relative mass of 1. (review the periodic table to view the elements) a. Which atom is most likely to accept electrons to form an ionic bond? (a) ar (b) br (c) mg (d) p (e) k. Web note the usefulness of the periodic table in predicting likely ion formation and charge (figure 2.29). You'll get a detailed solution from a subject matter expert that helps you. For example, fluorine has seven valence. Web what atom is most likely to form a ion? Ad browse & discover thousands of science book titles, for less. Web the ions that we have discussed so far are called monatomic ions, that is, they are ions formed from only one atom. The most likely element to form a 1+ ion in ionic compound is. A) ar b) br c) mg d) p e) k.PPT 1 Name the ions formed by these elements and classify them as
What are difference between atom and ion? Definition, Types and
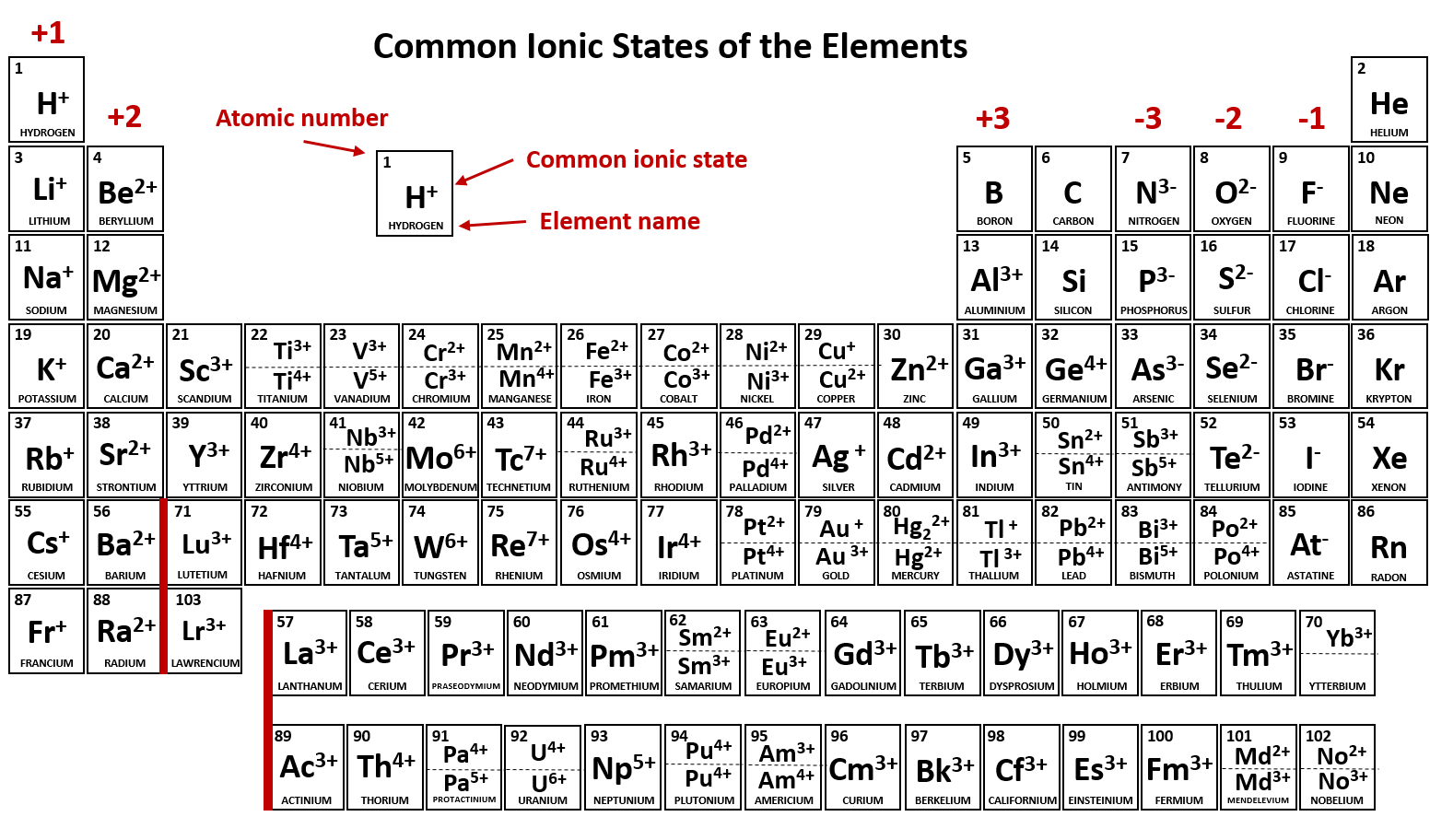
The Temptation News periodic table of elements with charges of ions
Which one of the atoms shown would most likely form a cation with a
CH104 Chapter 3 Ions and Ionic Compounds Chemistry
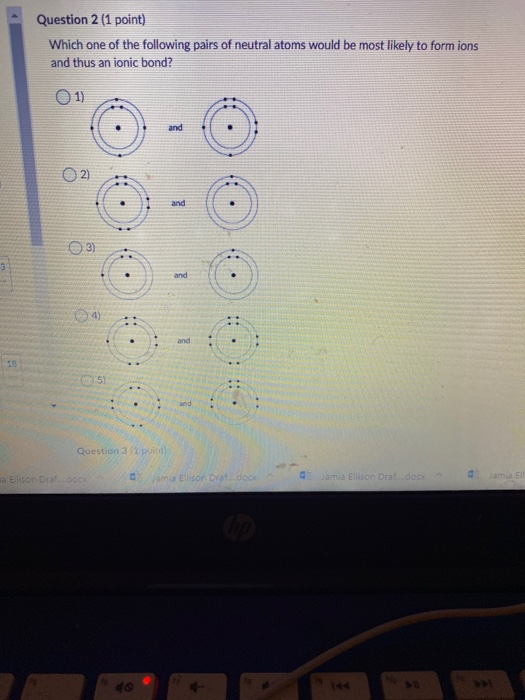
Solved Question 2 (1 point) Which one of the following pairs
Ionic Bond Definition, Types, Properties & Examples
Ionic Bonding Presentation Chemistry
PPT Test Review PowerPoint Presentation, free download ID2281673
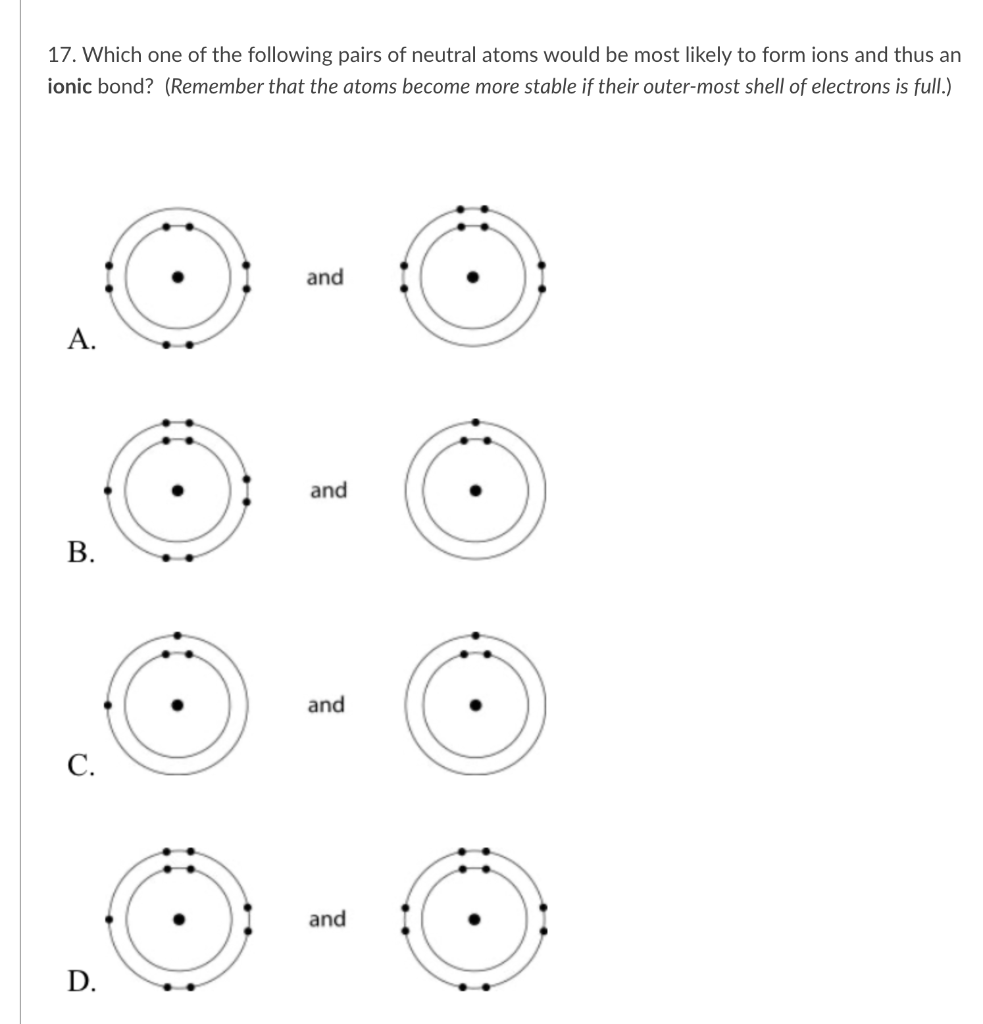
Solved 17. Which one of the following pairs of neutral atoms
Related Post:





.PNG)