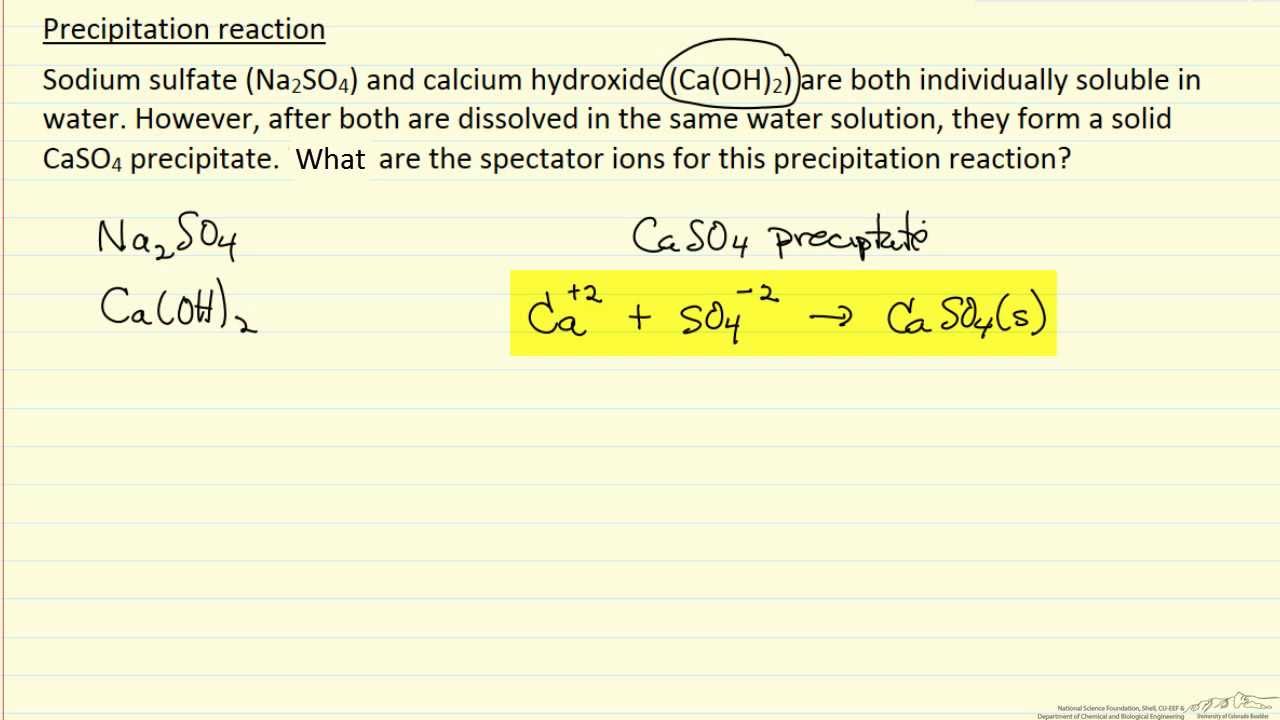Which Anion Will Form A Precipitate With Al3+
Which Anion Will Form A Precipitate With Al3+ - Web can sulfate be used to separate silver and lead ions? Web note that ammonia (nh 3) dissolves in water to produce a small concentration of hydroxide ions (discussed in a later section.) the resulting hydroxide ions can participate in. What is the \(\ce{[pb^2+]}\) when. If no precipitate forms, you must write nr. Web firstly, we want to write the equation for the reaction that occur to increase the soil ability off aluminum hydroxide. Web when a metal ion or a group of metal ions form insoluble salts with a particular anion, they can be separated from others by precipitation. We can also separate the anions by. Al3+ (aq) + 3no− 3(aq) + 3na+ (aq) + 3oh− (aq) → al(oh)3(s) ⏐ ⏐↓ + 3na+ (aq) + 3no− 3(aq) to. Web precipitation reactions occur when two aqueous solutions are combined, causing a chemical reaction that produces a solid product. The ions present in the solutions. Web which pair of ions would not be expected to form a precipitate when solutions are mixed? Aluminium, al 3+ white precipitate: Web a precipitate will form if a solution containing one of these anions is added to a solution containing a metal cation such as fe2+, cu2+, or al3+. Al 3+ (aq) + 3nh 3 (aq)+ 3h 2 o. Aluminium, al 3+ white precipitate: Ba 2+ ion form precipitates with anions such as sulfate, sulfite and carbonate. Web can sulfate be used to separate silver and lead ions? Although this reaction is not suitable for separation of aluminum ion, it can be used as a confirmatory test for al3+ al3+ after precipitation of al(oh)3 al(oh)3 with aqueous ammonia. Al. Web when a metal ion or a group of metal ions form insoluble salts with a particular anion, they can be separated from others by precipitation. Web chemistry chemistry questions and answers question 5 (10 points) which anion will form a precipitate with a13+? Web note that ammonia (nh 3) dissolves in water to produce a small concentration of hydroxide. Al3+ (aq) + 3no− 3(aq) + 3na+ (aq) + 3oh− (aq) → al(oh)3(s) ⏐ ⏐↓ + 3na+ (aq) + 3no− 3(aq) to. The ions present in the solutions. Although this reaction is not suitable for separation of aluminum ion, it can be used as a confirmatory test for al3+ al3+ after precipitation of al(oh)3 al(oh)3 with aqueous ammonia. Web which. If no precipitate forms, you must write nr. The ions present in the solutions. 1.write the net ionic equation of your metal cation. Web can sulfate be used to separate silver and lead ions? So in acidic solution, it will be you. If no precipitate forms, you must write nr. What is the \(\ce{[pb^2+]}\) when. Web zn2+ ions will react to produce a white precipitate of zinc hydroxide, and we get a similar reaction with al3+ ions, producing aluminum hydroxide. 1.write the net ionic equation of your metal cation. Web firstly, we want to write the equation for the reaction that occur. Web chemistry chemistry questions and answers question 5 (10 points) which anion will form a precipitate with a13+? Ba 2+ ion form precipitates with anions such as sulfate, sulfite and carbonate. Al 3+ (aq) + 3nh 3 (aq)+ 3h 2 o (aq) <==> al (oh) 3 (s) + 3nh 4+ (aq) sodium. Question 5 why doesn't al3+ form a precipitate. Web aluminum ion reacts with aqueous ammonia to produce a white gelatinous precipitate of al (oh) 3: So in acidic solution, it will be you. Web can sulfate be used to separate silver and lead ions? Web note that ammonia (nh 3) dissolves in water to produce a small concentration of hydroxide ions (discussed in a later section.) the resulting. Web the complete ionic equation for this reaction looks like this. Web firstly, we want to write the equation for the reaction that occur to increase the soil ability off aluminum hydroxide. 1.write the net ionic equation of your metal cation. We can also separate the anions by. Web chemistry chemistry questions and answers question 5 (10 points) which anion. We can also separate the anions by. Although this reaction is not suitable for separation of aluminum ion, it can be used as a confirmatory test for al3+ al3+ after precipitation of al(oh)3 al(oh)3 with aqueous ammonia. Web can sulfate be used to separate silver and lead ions? Web which anion will form a precipitate with ba2+? The product is. Web chemistry chemistry questions and answers question 5 (10 points) which anion will form a precipitate with a13+? Web zn2+ ions will react to produce a white precipitate of zinc hydroxide, and we get a similar reaction with al3+ ions, producing aluminum hydroxide. Which one will form a precipitate first as the sulfate ion concentration increases? The ions present in the solutions. What is the \(\ce{[pb^2+]}\) when. Web when a metal ion or a group of metal ions form insoluble salts with a particular anion, they can be separated from others by precipitation. Question 5 why doesn't al3+ form a precipitate when reacted with naoh? 1.write the net ionic equation of your metal cation. Al 3+ (aq) + 3nh 3 (aq)+ 3h 2 o (aq) <==> al (oh) 3 (s) + 3nh 4+ (aq) sodium. If no precipitate forms, you must write nr. The product is al (oh)3 which is soluble in water. Aluminium, al 3+ white precipitate: Ba 2+ ion form precipitates with anions such as sulfate, sulfite and carbonate. Al3+ (aq) + 3no− 3(aq) + 3na+ (aq) + 3oh− (aq) → al(oh)3(s) ⏐ ⏐↓ + 3na+ (aq) + 3no− 3(aq) to. Web can sulfate be used to separate silver and lead ions? Web aluminum ion reacts with aqueous ammonia to produce a white gelatinous precipitate of al (oh) 3: We can also separate the anions by. Therefore, barium carbonate, barium sulfate and. Web which anion will form a precipitate with ba2+? Web a precipitate will form if a solution containing one of these anions is added to a solution containing a metal cation such as fe2+, cu2+, or al3+.PPT Formation of a precipitate PowerPoint Presentation, free download
Periodic Table List Of Cations And Anions Periodic Table Timeline
Pin on Quimica
Solubility of Anions and Cations Information Sheet Teaching Resources
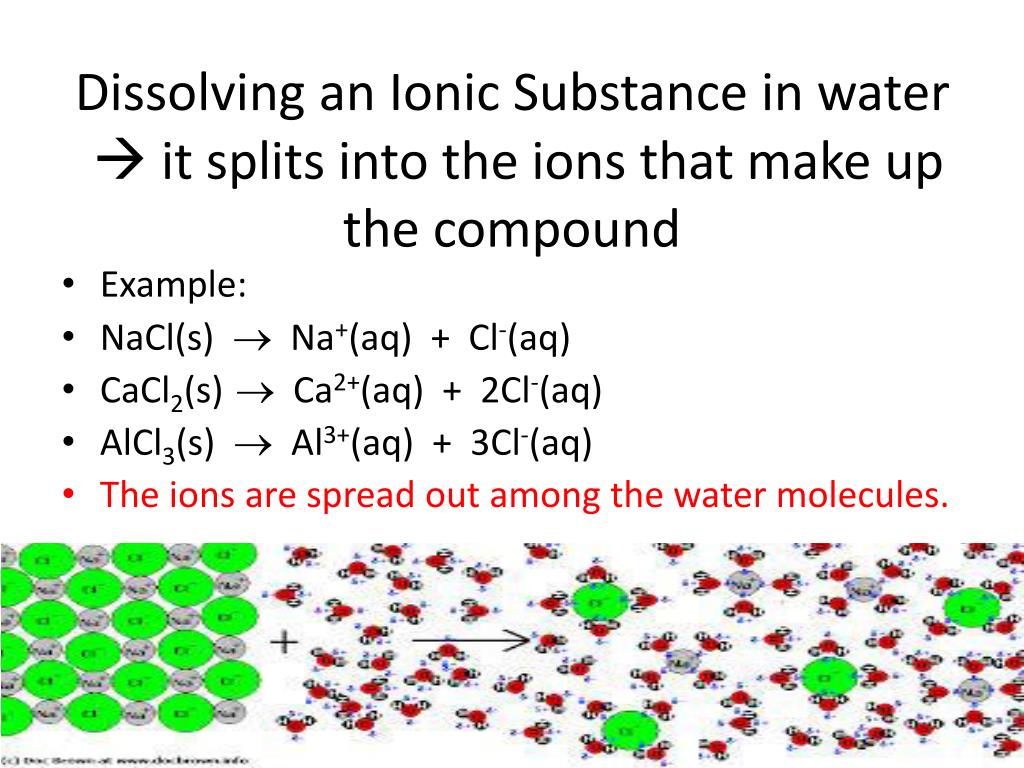
Solved For the Ag cation, choose the formulas of the anions
Solved Part 2(1 pt) See Hin Which cation and which anion
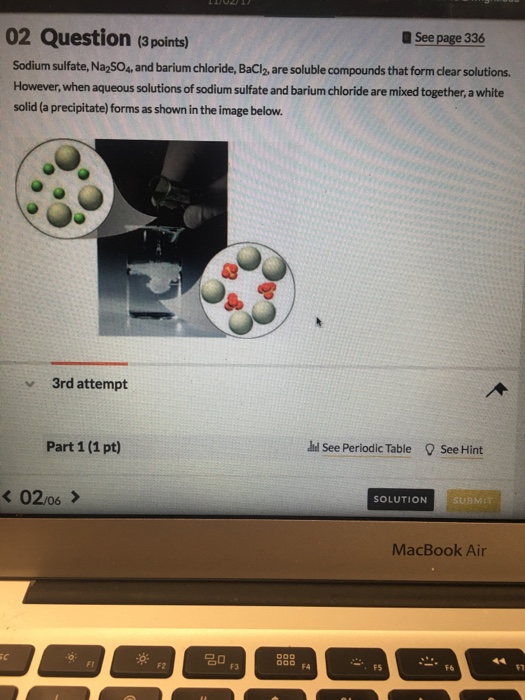
Solved Below is a list of cations and anions . Write the
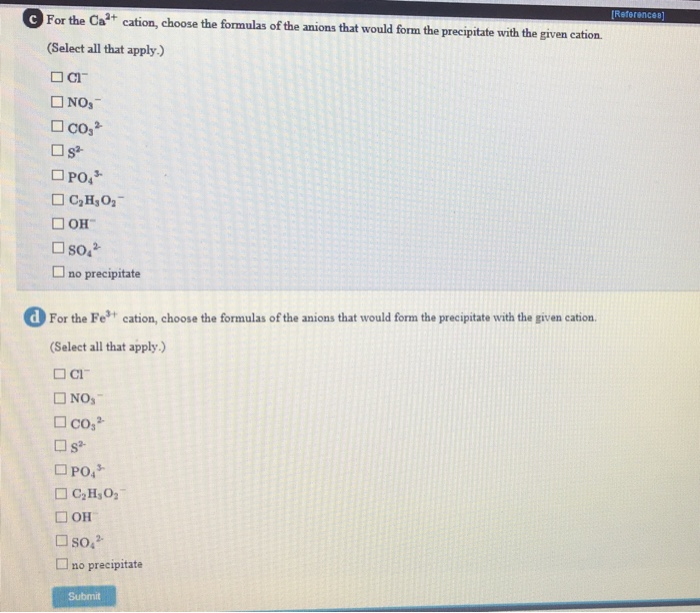
Precipitation Reaction (Spectator Ions) (Example) YouTube
cations and anions table Chemistry, Calculus, Lesson
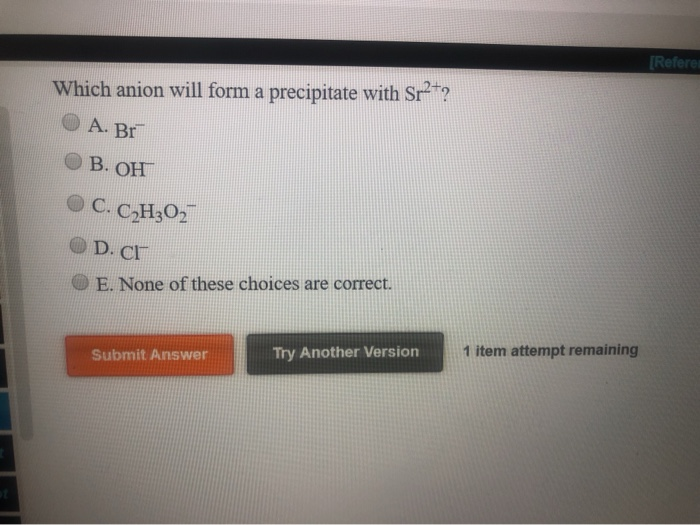
Solved [Referen Which anion will form a precipitate with
Related Post: