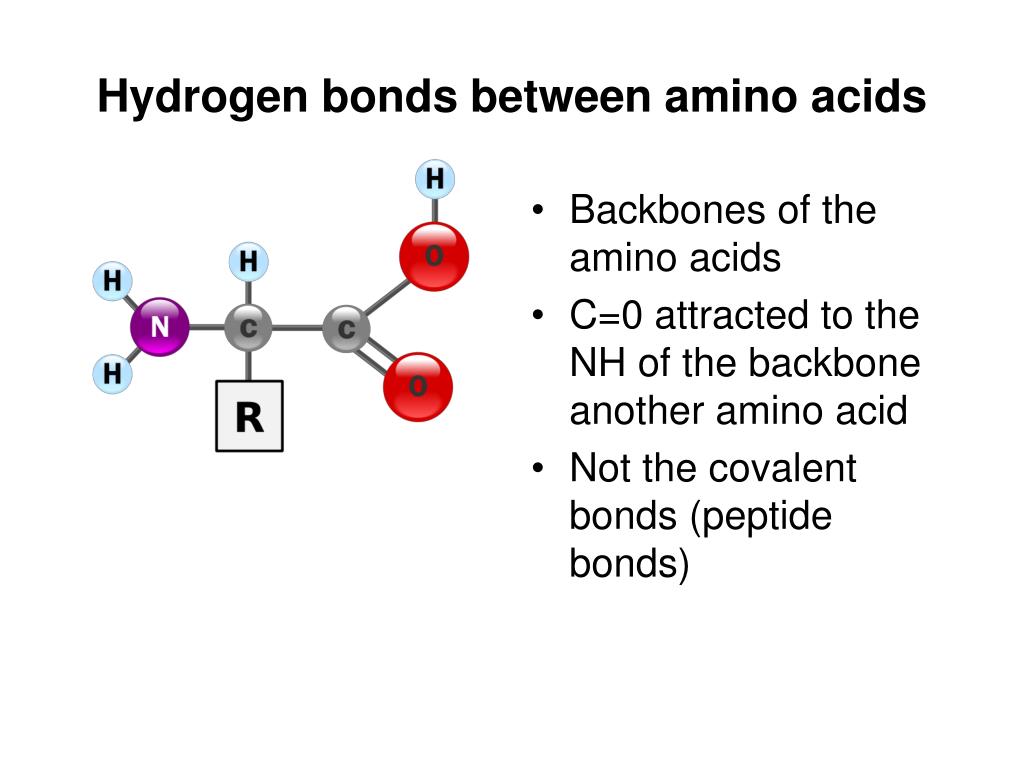
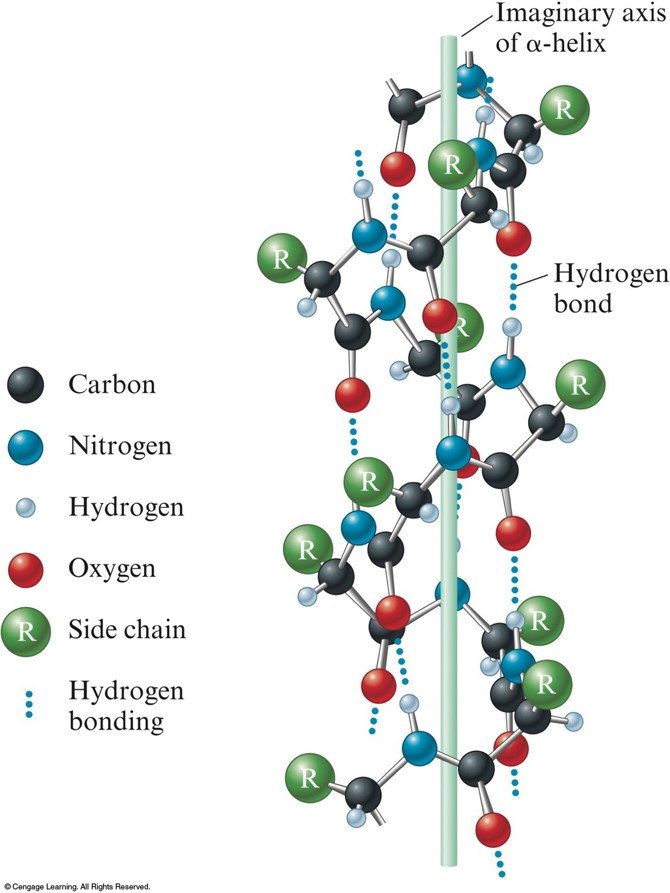
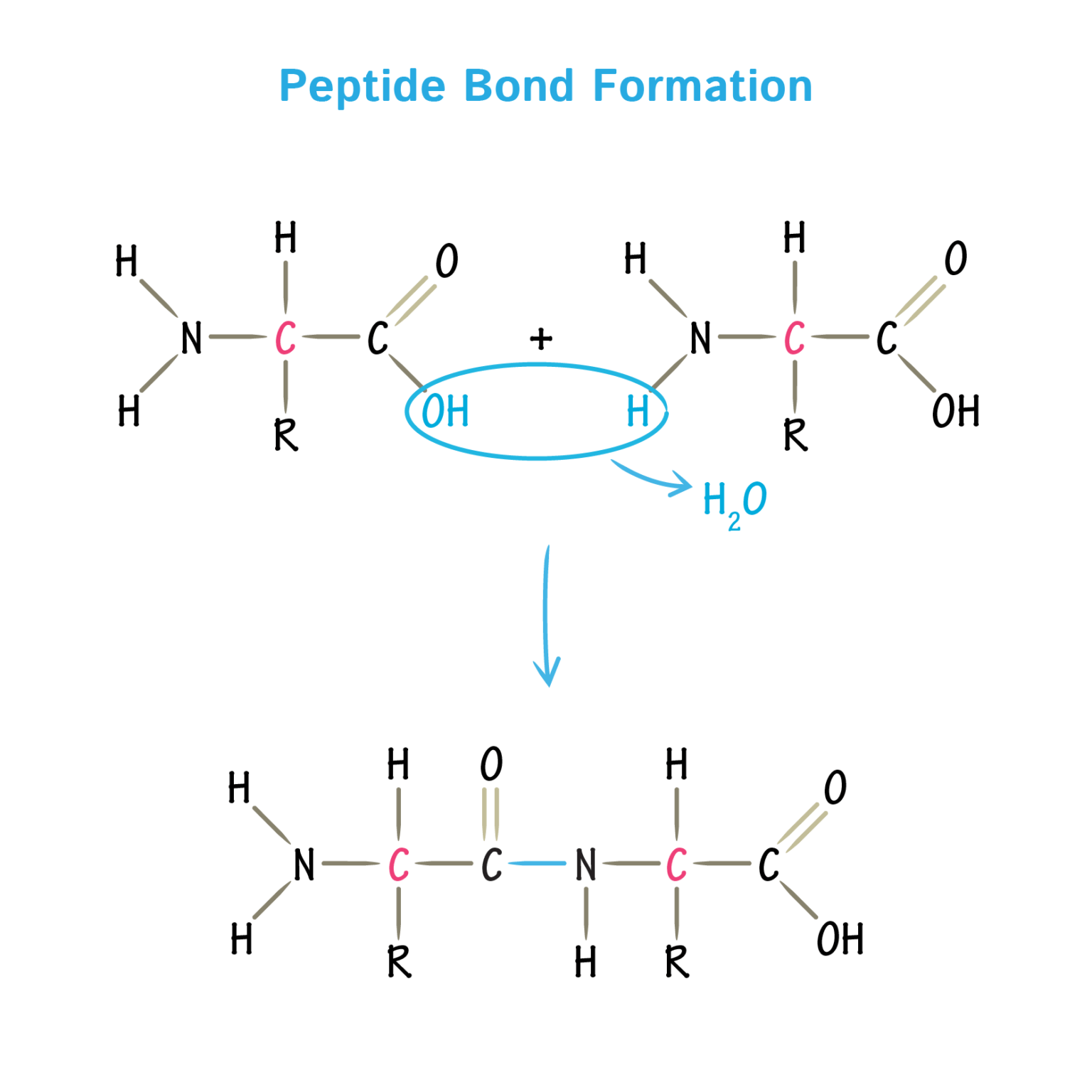
Which Amino Acids Can Form Hydrogen Bonds
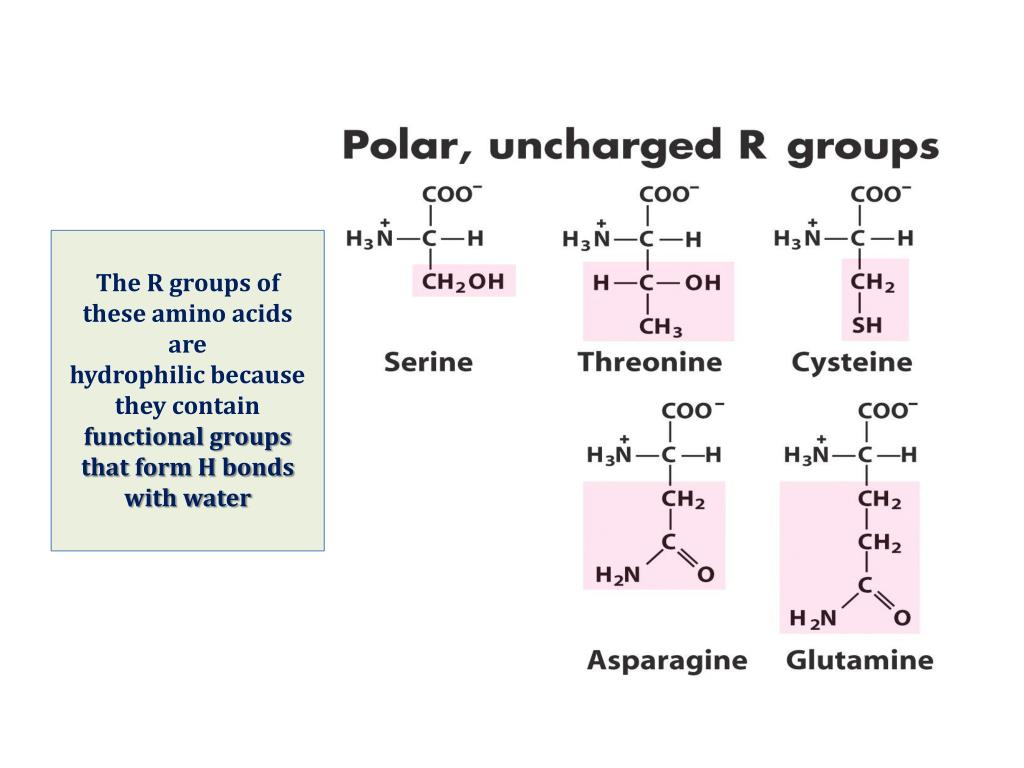
Which Amino Acids Can Form Hydrogen Bonds - Amino acids can be linked by a condensation reaction in which an ―oh is lost from the carboxyl group of one amino acid along with a hydrogen from the amino group of a second, forming a molecule of water and leaving the two… hydrogen bonds. The amino acids lysine, arginine, and histidine have side chains with charged basic groups. This is an example of severe perturbation, and is not. Web because the polar side chains of these amino acids can form hydrogen bonds with water, these amino acids are hydrophilic and tend to be located on the outside of proteins. Web there are 20 types of amino acids commonly found in proteins. Web polar amino acids (form hydrogen bonds as proton donors or acceptors): Hydrophobic side chains interact with each other via weak van der waals. Molecule which bears charged groups of opposite polarity. The amino acids are joined together by structures called peptide bonds. What amino acid participated in disulfide bonds? The nonessential amino acids are alanine, asparagine, aspartic acid, glutamic acid, and serine. Molecule which bears charged groups of opposite polarity. The amino acids are joined together by structures called peptide bonds. Such a hydrogen bond is formed exactly every 4 amino acid residues, and every complete turn of the helix is only 3.6 amino acid residues. Such a bond. Web which amino acids can form hydrogen bonds. Such a bond is weaker than an ionic bond or covalent bond but stronger than van der waals forces.hydrogen bonds can exist between atoms in different molecules or in parts of the same molecule. Molecule which bears charged groups of opposite polarity. The amino acids lysine, arginine, and histidine have side chains. There are two requirements for hydrogen bonding. The forces in secondary structure primarily involve hydrogen bonds. Web polar amino acids (form hydrogen bonds as proton donors or acceptors): In this rotating model oxygen are red, carbon grey and hydrogen white. Hydrogen bonds, ionic bonds, and van der waals. There are two requirements for hydrogen bonding. Amino acids can be linked by a condensation reaction in which an ―oh is lost from the carboxyl group of one amino acid along with a hydrogen from the amino group of a second, forming a molecule of water and leaving the two… hydrogen bonds. (figure 1) draw it as it would. Web. This problem has been solved! Amino acids can be linked by a condensation reaction in which an ―oh is lost from the carboxyl group of one amino acid along with a hydrogen from the amino group of a second, forming a molecule of water and leaving the two… hydrogen bonds. Such a hydrogen bond is formed exactly every 4 amino. Web because the polar side chains of these amino acids can form hydrogen bonds with water, these amino acids are hydrophilic and tend to be located on the outside of proteins. Web these include peptide bonds, which link amino acids together in a linear sequence. This problem has been solved! A few biologically important derivatives of the standard amino acids. In this rotating model oxygen are red, carbon grey and hydrogen white. This is an example of severe perturbation, and is not. Web example of salt bridge between amino acids glutamic acid and lysine demonstrating electrostatic interaction and hydrogen bonding. Web 1 day agoscience biochemistry proteins are made from chains of amino acids. Amino acids can be linked by a. X ray data indicate that this helix makes one turn for every 3.6 amino acids, and the side. Conditional amino acids include arginine, cysteine, glutamine, glycine, proline, and tyrosine. There are two requirements for hydrogen bonding. Web which amino acids can form hydrogen bonds? Web charged amino acid side chains can form ionic bonds, and polar amino acids are capable. Amino acids can be linked by a condensation reaction in which an ―oh is lost from the carboxyl group of one amino acid along with a hydrogen from the amino group of a second, forming a molecule of water and leaving the two… hydrogen bonds. Amino acids share a basic structure, which consists of a central carbon atom, also known. The nonessential amino acids are alanine, asparagine, aspartic acid, glutamic acid, and serine. Such a bond is weaker than an ionic bond or covalent bond but stronger than van der waals forces.hydrogen bonds can exist between atoms in different molecules or in parts of the same molecule. Which amino acids are involved in turns and kinks? Amino acids share a. Amino acids share a basic structure, which consists of a central carbon atom, also known as the alpha (α) carbon, bonded to an amino group ( nh 2 ), a carboxyl group ( cooh ), and a hydrogen atom. They play an extensive role in gene expression process, which includes an adjustment of protein functions that facilitate messenger rna (mrna) translation (scot et al., 2006). Web example of salt bridge between amino acids glutamic acid and lysine demonstrating electrostatic interaction and hydrogen bonding. In this rotating model oxygen are red, carbon grey and hydrogen white. Amino acids can be linked by a condensation reaction in which an ―oh is lost from the carboxyl group of one amino acid along with a hydrogen from the amino group of a second, forming a molecule of water and leaving the two… hydrogen bonds. The weak bonds are of three types: Web figure 11.5.1 11.5. Web the hydrogen bonds form between the oxygen atom in the carbonyl group in one amino acid and another amino acid that is four amino acids farther along the chain. Web these include peptide bonds, which link amino acids together in a linear sequence. A) arginine and glutamic acidb) glutamine and serinec) aspartic acid and lysine d) leucine and alaninee) glycine and asparagineanswer: The forces in secondary structure primarily involve hydrogen bonds. (figure 1) draw it as it would. Part a draw the dipeptide that results when a peptide bond is formed between the two glycine molecules shown here. Such a bond is weaker than an ionic bond or covalent bond but stronger than van der waals forces.hydrogen bonds can exist between atoms in different molecules or in parts of the same molecule. The amino acids are joined together by structures called peptide bonds. Such a hydrogen bond is formed exactly every 4 amino acid residues, and every complete turn of the helix is only 3.6 amino acid residues. Tyrosine possesses a hydroxyl group in the aromatic ring, making it a phenol derivative. X ray data indicate that this helix makes one turn for every 3.6 amino acids, and the side. Amino acids are a crucial, yet basic unit of protein, and they contain an amino group and a carboxylic group. The nonessential amino acids are alanine, asparagine, aspartic acid, glutamic acid, and serine.PPT Inner Life of a Cell PowerPoint Presentation, free download ID
PPT Proteins PowerPoint Presentation, free download ID1828850
organic chemistry Which atoms in a given amino acid are able to form
amino acids salt bridge vs hydrogen bond Chemistry Stack Exchange
Chapter 22 Presentation
Amino acids physical, chemical properties and peptide bond
(a) Hydrogen bonding patterns that describe the αand πconfigurations
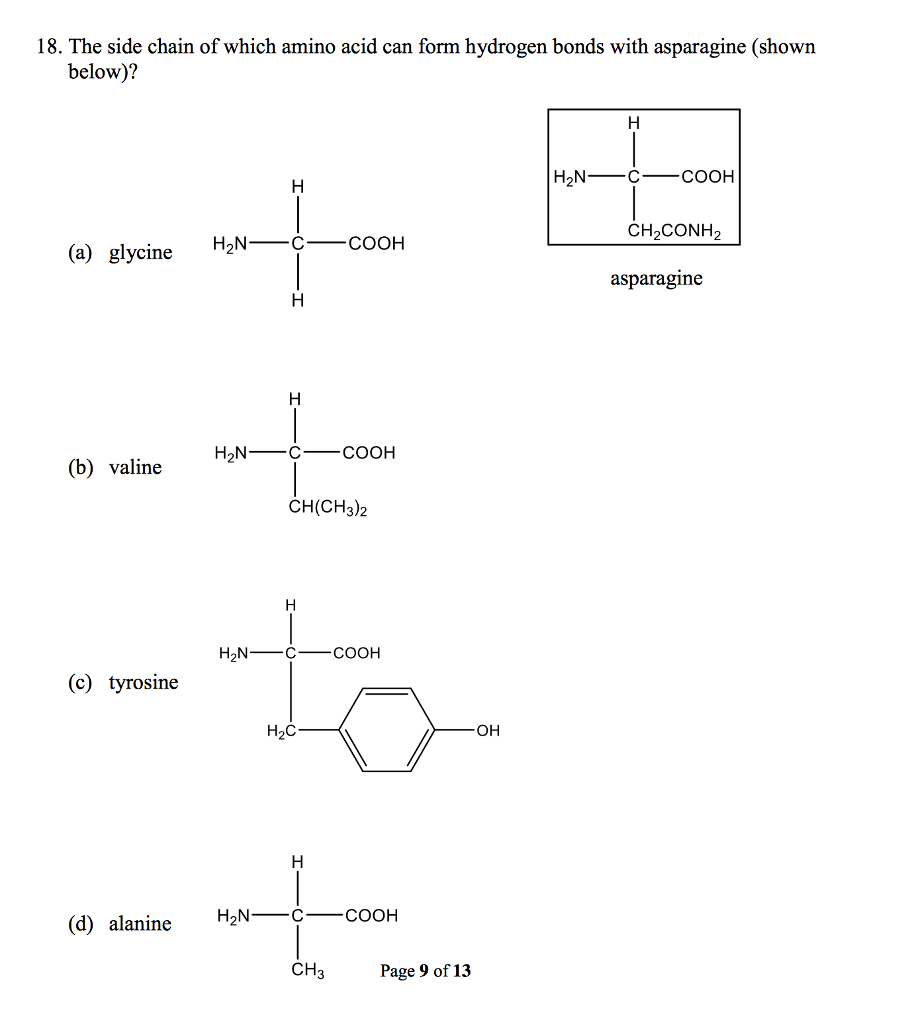
Solved 18. The side chain of which amino acid can form
PPT Introduction to Amino Acids of Medical Importance PowerPoint
Two amino acids are joined together by
Related Post: