What Type Of Ions Do Nonmetals Form
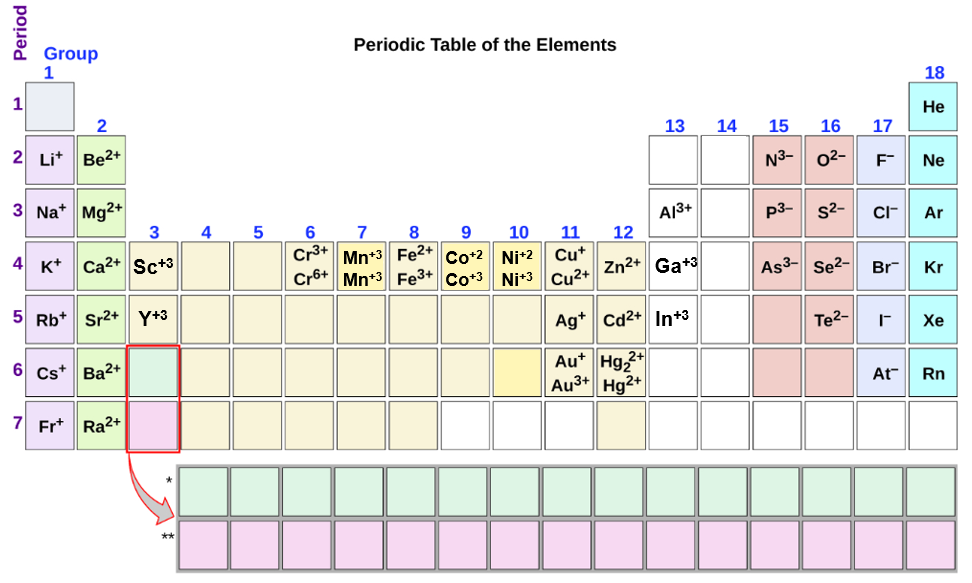
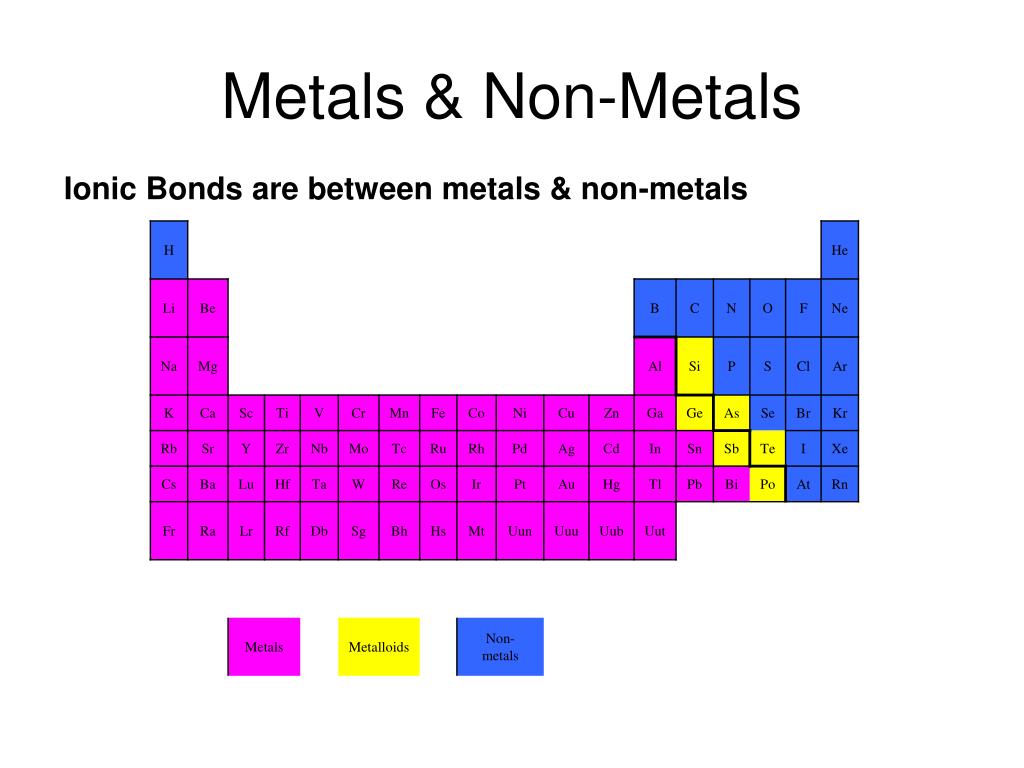
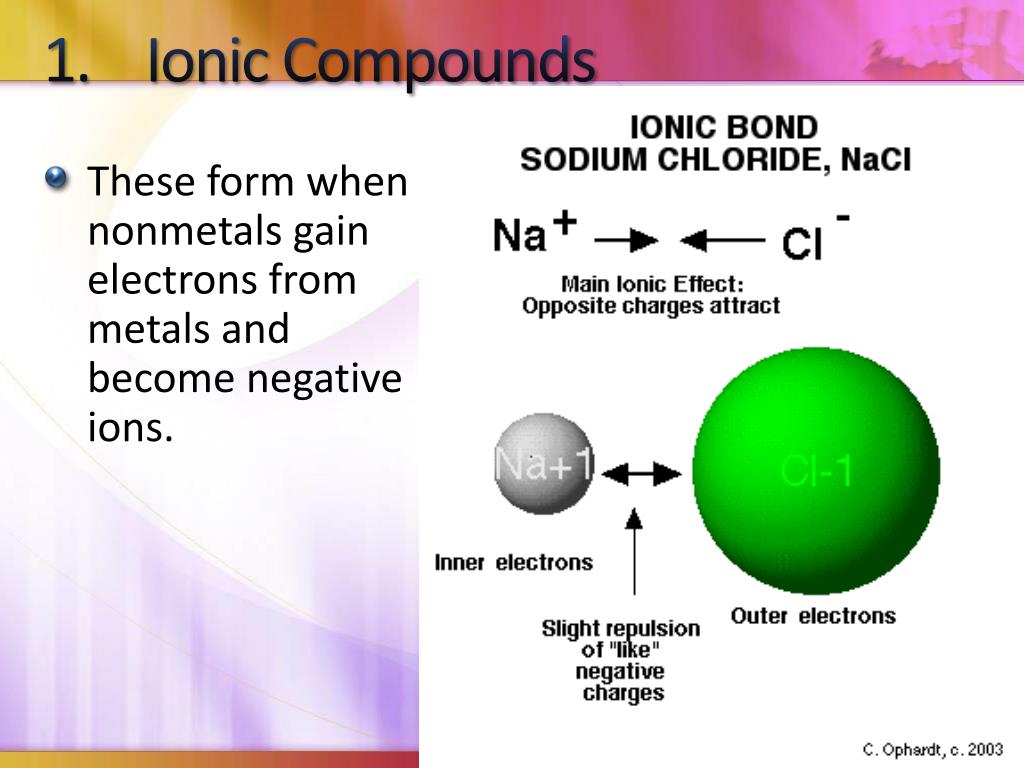
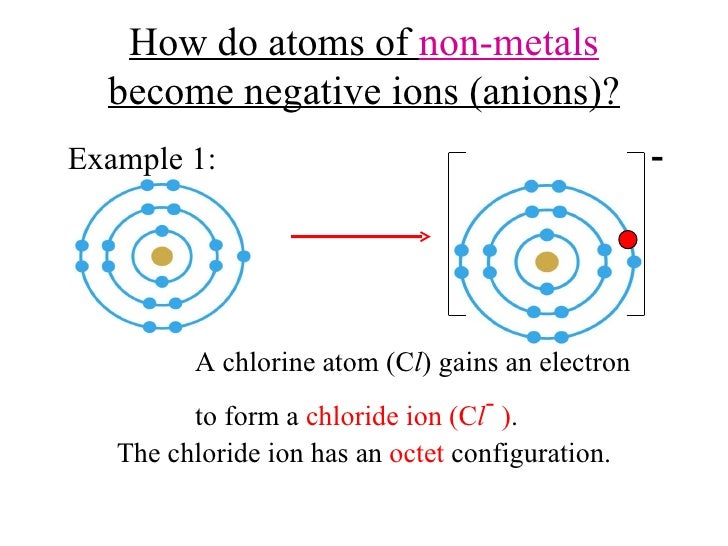
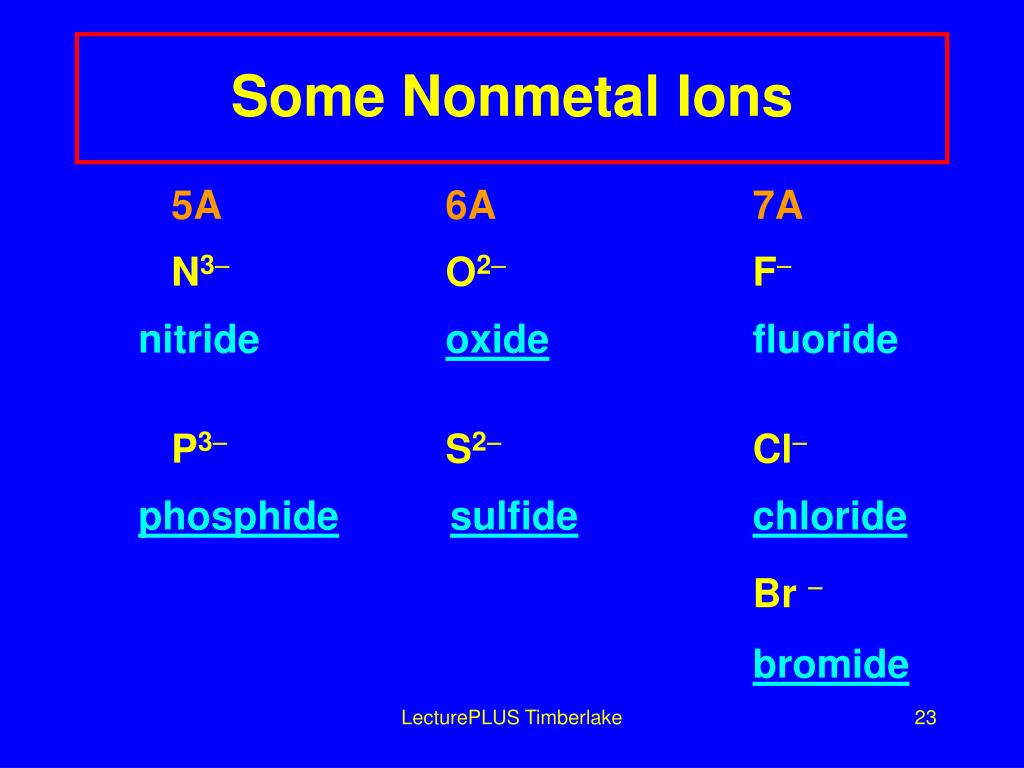
What Type Of Ions Do Nonmetals Form - Web we would like to show you a description here but the site won’t allow us. Of attraction between the oppositely charged ions hold them together. Nonmetals form negatively charged ions, or anions. Metals tend to have low. These are electronegative elements with high ionization energies. Negative ions, by gaining electrons to fill the valence shell. So, the correct option is option a. Web the type of ions that nonmetals naturally form are negative ions, by gaining electrons to fill the valence shell. They do this because they need to gain one to three electrons in order to. Sulfur is dull yellow and very brittle. What is the formula of the compound formed between chlorine (cl). Of attraction between the oppositely charged ions hold them together. The common oxidation states that the nonmetals. Metals tend to have low. Web when nonmetals form ions, what type of ions do they tend to form? Web when nonmetals form ions, what type of ions do they tend to form? First, each element that forms cations is a metal, except for one (hydrogen), while each element that. Web all monatomic nonmetal ions are anions; The common oxidation states that the nonmetals. Web the type of ions that nonmetals naturally form are negative ions, by gaining electrons. Web the chemical differences between metals and nonmetals that interest us the most: Web we would like to show you a description here but the site won’t allow us. When it reacts with other elements, it tends to form s2− ions. The ionization energy of an element describes the amount of energy needed to cause an atom to lose an. Web all monatomic nonmetal ions are anions; Positively charged ions are called cations, and negatively charged ions are called anions. Web the chemical differences between metals and nonmetals that interest us the most: Web when nonmetals form ions, what type of ions do they tend to form? They do this because they need to gain one to three electrons in. Web all monatomic nonmetal ions are anions; Web there are several things to notice about the ions in figure \(\pageindex{1}\). Web thus, nonmetals tend to form negative ions. Examples include the chloride ion, cl −, the nitride ion, n 3−, and the selenide ion, se 2−. Of attraction between the oppositely charged ions hold them together. Web what type of ions do nonmetals naturally form? Sulfur is dull yellow and very brittle. The ionization energy of an element describes the amount of energy needed to cause an atom to lose an electron. Examples include the chloride ion, cl −, the nitride ion, n 3−, and the selenide ion, se 2−. Web the type of ions that. These are electronegative elements with high ionization energies. What is the formula of the compound formed between chlorine (cl). Web thus, nonmetals tend to form negative ions. Web all monatomic nonmetal ions are anions; Sulfur is dull yellow and very brittle. Of attraction between the oppositely charged ions hold them together. Web we would like to show you a description here but the site won’t allow us. Ions can be either monatomic (containing only. Web when nonmetals form ions, what type of ions do they tend to form? Web all monatomic nonmetal ions are anions; Web all monatomic nonmetal ions are anions; Sulfur is dull yellow and very brittle. Web thus, nonmetals tend to form negative ions. Of attraction between the oppositely charged ions hold them together. Web when nonmetals form ions, what type of ions do they tend to form? Sulfur is dull yellow and very brittle. Web when nonmetals form ions, what type of ions do they tend to form? Negative ions, by gaining electrons to fill the valence shell. They do this because they need to gain one to three electrons in order to. The common oxidation states that the nonmetals. Nonmetals form negatively charged ions, or anions. When it reacts with other elements, it tends to form s2− ions. Negative ions, by gaining electrons to fill the valence shell. Sulfur is dull yellow and very brittle. Web when nonmetals form ions, what type of ions do they tend to form? Web what type of ions do nonmetals naturally form? Web thus, nonmetals tend to form negative ions. The ionization energy of an element describes the amount of energy needed to cause an atom to lose an electron. Web all monatomic nonmetal ions are anions; Of attraction between the oppositely charged ions hold them together. These are electronegative elements with high ionization energies. Positively charged ions are called cations, and negatively charged ions are called anions. They do this because they need to gain one to three electrons in order to. Of attraction between the oppositely charged ions hold them together. Examples include the chloride ion, cl −, the nitride ion, n 3−, and the selenide ion, se 2−. Web there are several things to notice about the ions in figure \(\pageindex{1}\). Web what kind of ions do nonmetals form? Ions can be either monatomic (containing only. Web the chemical differences between metals and nonmetals that interest us the most: Web the type of ions that nonmetals naturally form are negative ions, by gaining electrons to fill the valence shell.Ionic Bond Definition, Types, Properties & Examples
4.3 Ionic Compounds and Formulas (2022)
PPT IONS PowerPoint Presentation, free download ID2435906
PPT Ionic Compounds Formula to Name PowerPoint Presentation, free
Chem matters ch6_ionic_bond
PPT Chapter 19 Elements And Their Properties PowerPoint Presentation
Chem matters ch6_ionic_bond
PPT Chemical Bonds PowerPoint Presentation, free download ID930911
Chem matters ch6_ionic_bond
Introductory Chemistry Nonmetals (Part 1)
Related Post: