What Type Of Bonds Does Carbon Form With Other Elements
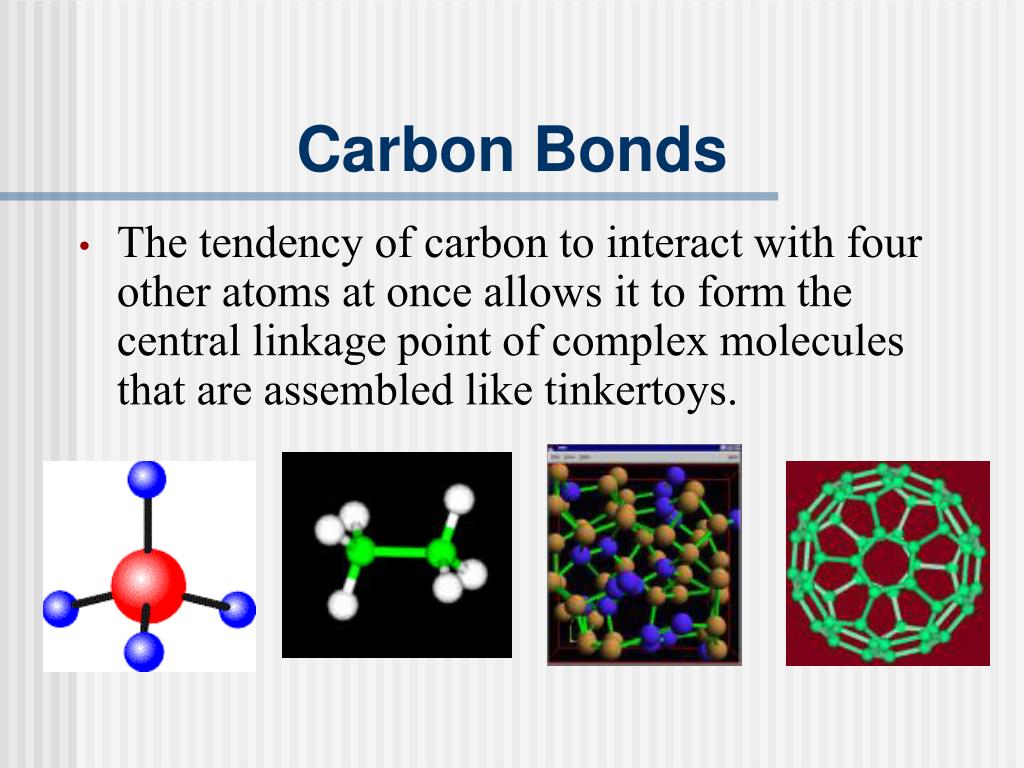
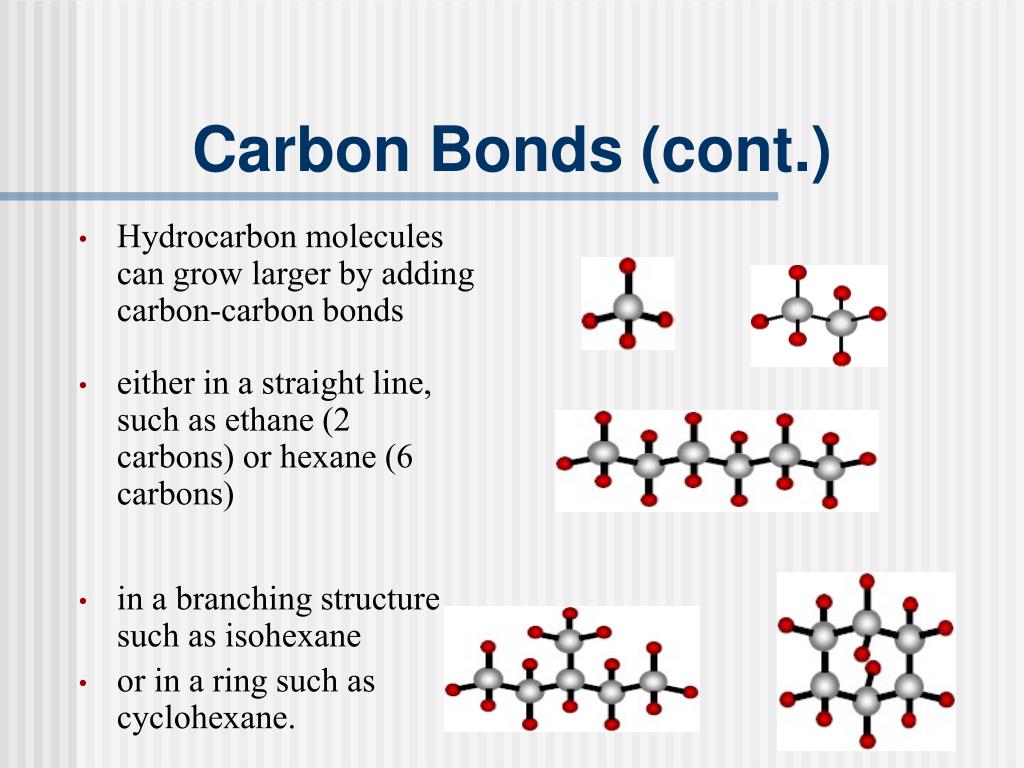
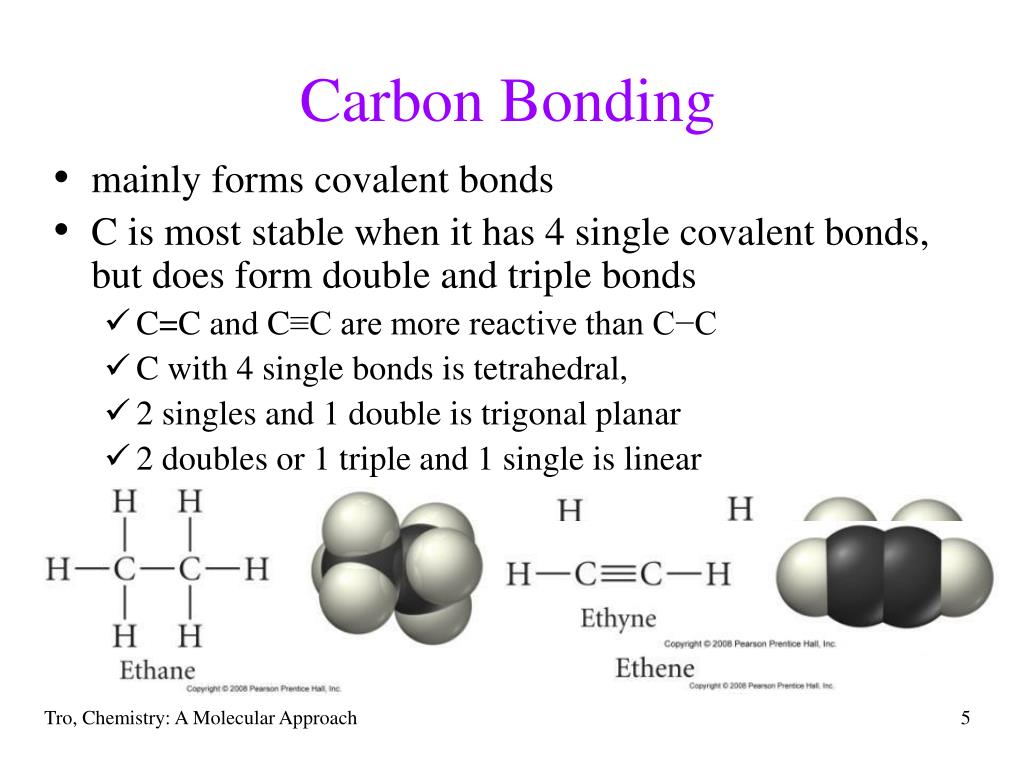
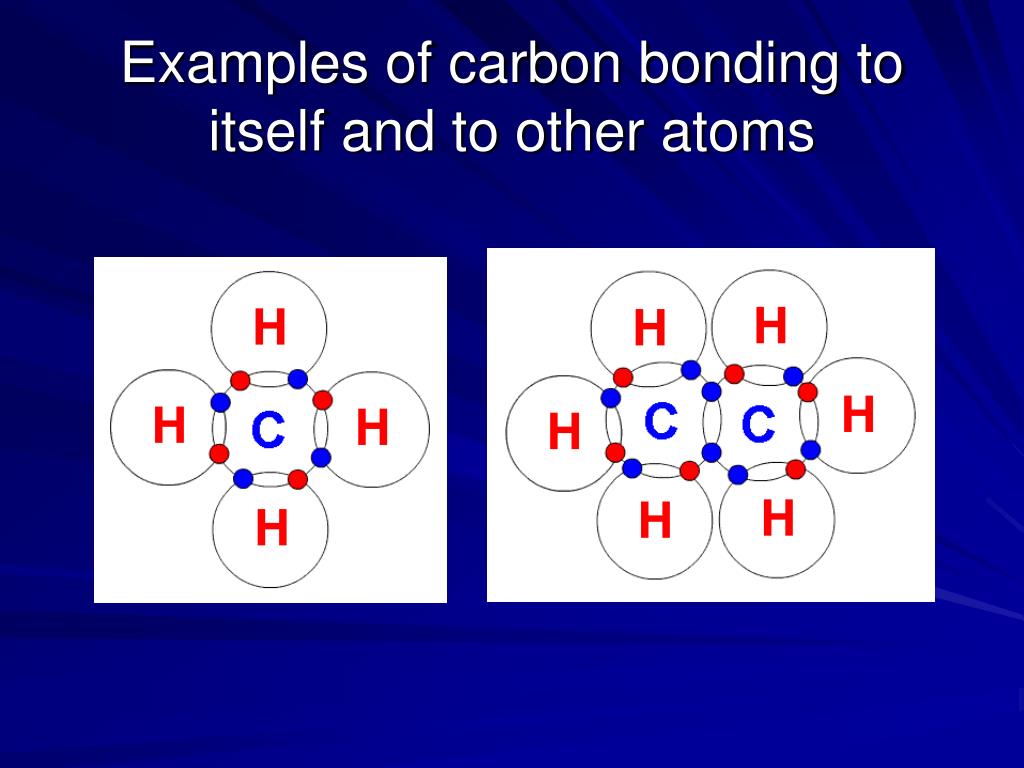
What Type Of Bonds Does Carbon Form With Other Elements - Carbon forms covalent bonds with atoms of carbon or other elements. Web carbon contains four electrons in its outer shell. Web one carbon atom forms four covalent bonds with four hydrogen atoms by sharing a pair of electrons between itself and each hydrogen (h) atom. In a double bond, they share two. Carbon’s ability to form bonds with four other atoms goes back to its number and configuration of electrons. To form ionic bonds, carbon molecules must either gain or. Web in general bonds of carbon with other elements are covalent bonds. Web these four electrons can be gained by forming four covalent bonds, as illustrated here for carbon in ccl 4 (carbon tetrachloride) and silicon in sih 4 (silane). Web a carbon atom can form covalent bonds with other carbon atoms or with the atoms of other elements. Web the carbon atoms may bond with atoms of other elements, such as nitrogen, oxygen, and phosphorus (figure 2 b ). Carbon most often forms a covalent bond with other atoms. Web therefore, carbon atoms can form up to four covalent bonds with other atoms to satisfy the octet rule. The simplest organic carbon molecule is methane (ch 4 ), in which four hydrogen atoms bind to a. Web one carbon atom forms four covalent bonds with four hydrogen atoms by. The molecules may also form rings, which themselves can. Carbon does not form ionic bonds because it has 4 valence electrons, half of an octet. Web moreover, of all the elements in the second row, carbon has the maximum number of outer shell electrons (four) capable of forming covalent bonds. To form ionic bonds, carbon molecules must either gain or.. Carbon often forms bonds with hydrogen. The simplest organic carbon molecule is. Carbon most often forms a covalent bond with other atoms. Therefore, it can form four covalent bonds with other atoms or molecules. To form ionic bonds, carbon molecules must either gain or. Carbon often forms bonds with hydrogen. In a single bond, two carbon atoms share one pair of electrons. If it is with another atom, a polar covalent bond is formed. The simplest organic carbon molecule is methane (ch 4 ), in which four hydrogen atoms bind to a. There is a great diversity of carbon compounds, ranging in size from. Web moreover, of all the elements in the second row, carbon has the maximum number of outer shell electrons (four) capable of forming covalent bonds. Carbon often forms bonds with hydrogen. Web dec 10, 2016. Carbon’s ability to form bonds with four other atoms goes back to its number and configuration of electrons. Web in general bonds of carbon with. Web these four electrons can be gained by forming four covalent bonds, as illustrated here for carbon in ccl 4 (carbon tetrachloride) and silicon in sih 4 (silane). Carbon most often forms a covalent bond with other atoms. Web carbon can form single, double, or even triple bonds with other carbon atoms. Web one carbon atom forms four covalent bonds. It has the chemical formula ch. Carbon forms covalent bonds with atoms of carbon or other elements. The simplest organic carbon molecule is methane (ch 4 ), in which four hydrogen atoms bind to a. If it is with another atom, a polar covalent bond is formed. Carbon most often forms a covalent bond with other atoms. The simplest organic carbon molecule is. Web therefore, carbon atoms can form up to four covalent bonds with other atoms to satisfy the octet rule. The molecules may also form rings, which themselves can. Generally, carbon atoms form single covalent bonds. Web carbon contains four electrons in its outer shell. Carbon’s ability to form bonds with four other atoms goes back to its number and configuration of electrons. In a single bond, two carbon atoms share one pair of electrons. Web carbon forms ionic bond with other elements if the electronegativity difference is more than 1.7 and covalent bond with other elements if the. Web a carbon atom can form. Web one carbon atom forms four covalent bonds with four hydrogen atoms by sharing a pair of electrons between itself and each hydrogen (h) atom. Web moreover, of all the elements in the second row, carbon has the maximum number of outer shell electrons (four) capable of forming covalent bonds. Carbon most often forms a covalent bond with other atoms.. If it is with another atom, a polar covalent bond is formed. Web therefore, carbon atoms can form up to four covalent bonds with other atoms to satisfy the octet rule. Carbon most often forms a covalent bond with other atoms. Carbon’s ability to form bonds with four other atoms goes back to its number and configuration of electrons. To form ionic bonds, carbon molecules must either gain or. Generally, carbon atoms form single covalent bonds. There is a great diversity of carbon compounds, ranging in size from just one to thousands of. Web carbon contains four electrons in its outer shell. Carbon forms covalent bonds with atoms of carbon or other elements. The bonds between these carbons are. The simplest organic carbon molecule is. In a single bond, two carbon atoms share one pair of electrons. The methane molecule provides an example: If the bond is with another carbon atom, it is a pure covalent (or nonpolar covalent) bond. The molecules may also form rings, which themselves can. Web carbon can form single, double, or even triple bonds with other carbon atoms. Web in general bonds of carbon with other elements are covalent bonds. Web carbon forms ionic bond with other elements if the electronegativity difference is more than 1.7 and covalent bond with other elements if the. Carbon does not form ionic bonds because it has 4 valence electrons, half of an octet. In a double bond, they share two.PPT Biochemistry PowerPoint Presentation, free download ID89333
PPT Biochemistry PowerPoint Presentation, free download ID89333
PPT Carbon Compounds PowerPoint Presentation, free download ID2319022
PPT Biochemistry PowerPoint Presentation, free download ID89333
PPT Unit 1 Biochemistry The Chemistry of Life PowerPoint
PPT Organic Chemistry Functional Groups PowerPoint Presentation
Four covalent bonds. Carbon has four valence electrons and here a
PPT Chapter 20 Organic Chemistry PowerPoint Presentation, free
Carbon to Carbon Single, Double & Triple Bonds Surfguppy
PPT The Molecules of Cells PowerPoint Presentation, free download
Related Post: