What Orbitals Form Pi Bonds
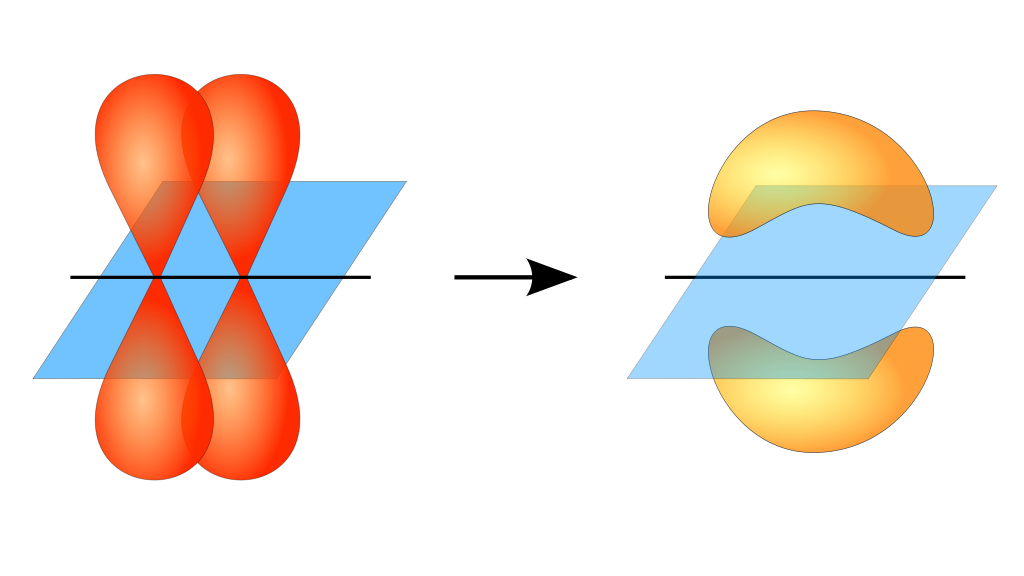
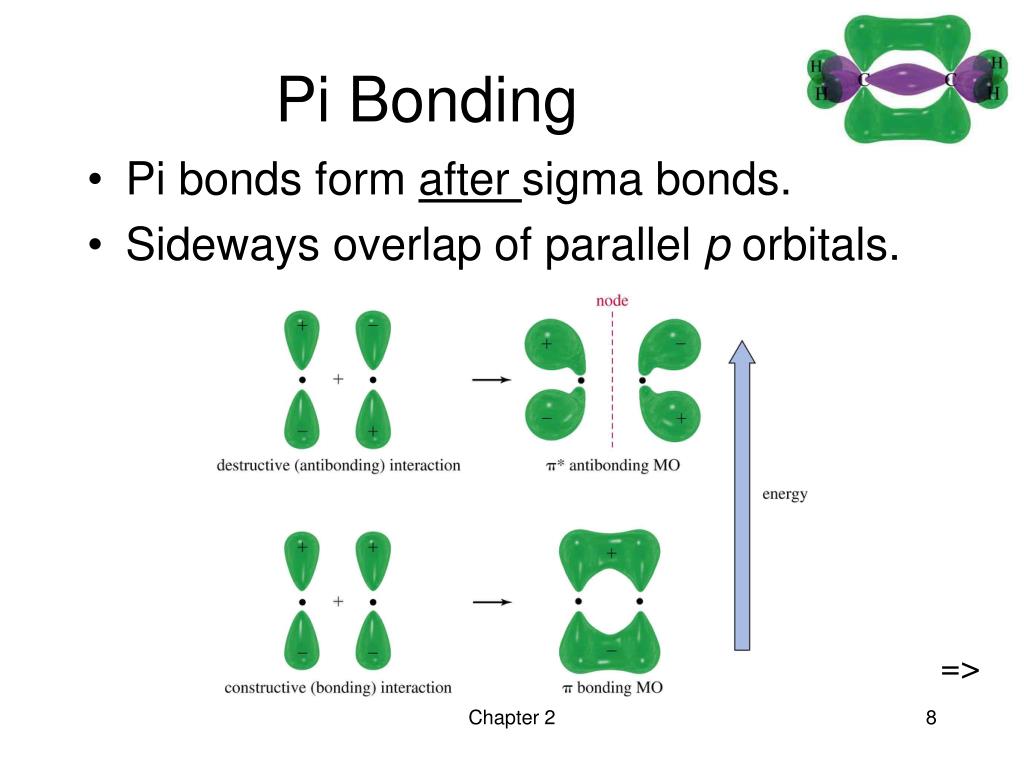
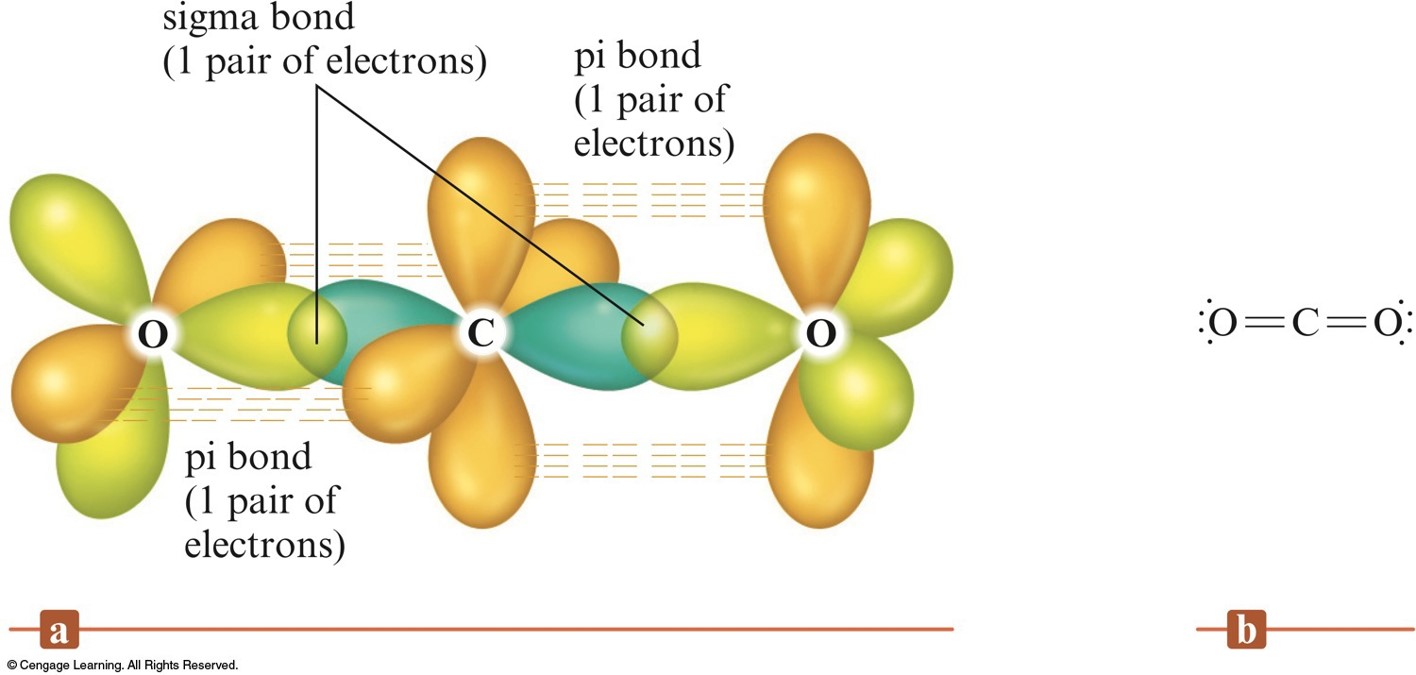
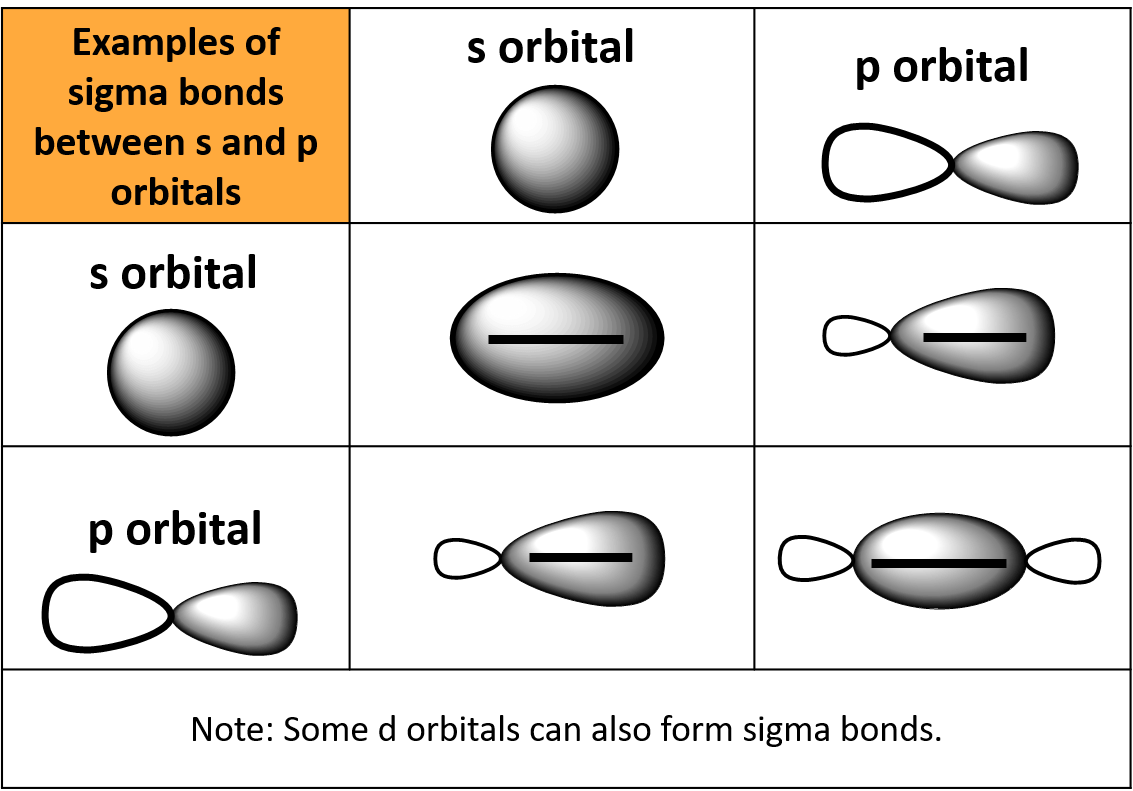
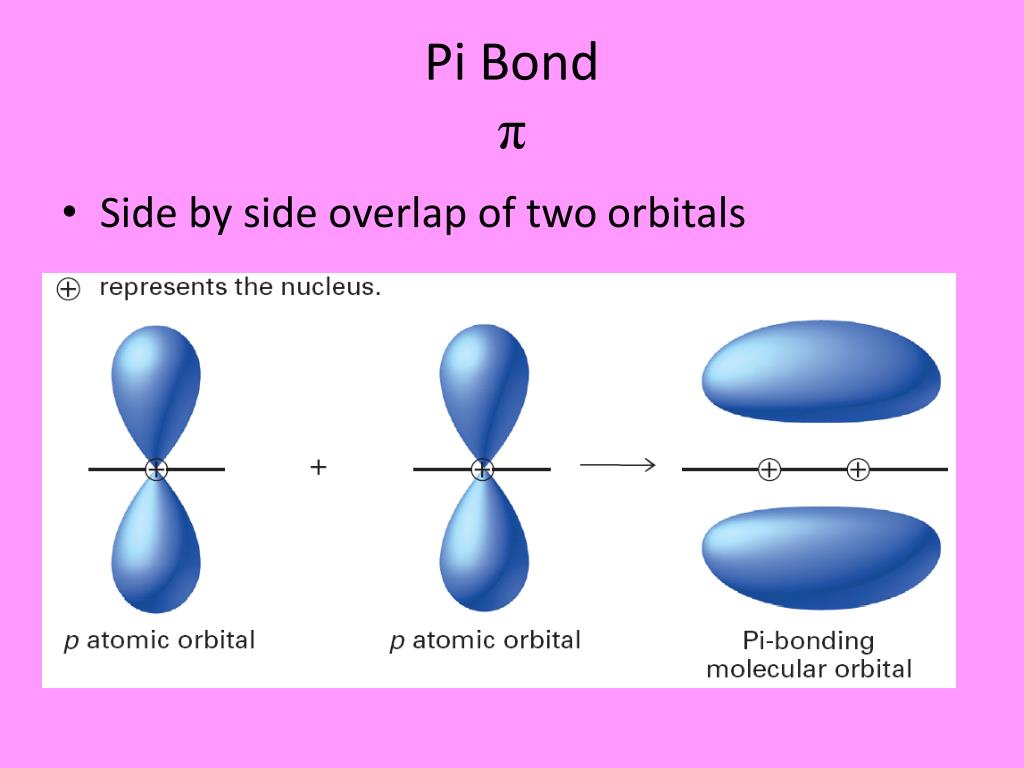
What Orbitals Form Pi Bonds - The most common types are formed by the overlap of p orbitals (fig. Web the three hybrid orbitals are used to form three sigma bonds, two with the two hydrogen atoms and one with the other carbon atom. Pi bonds are formed by side ways overlap of p orbital which leads lower energy state. Web what is the lewis structure of so2? Neither atomic orbitals nor molecular orbitals really exist so they. Web a pi bond is formed on the orbital axis due to the overlapping structure of orbital atoms. As you may see, there is a sideways overlap of the dumbbell shaped p orbitals in the. Web atomic orbitals forming molecular orbitals are a way of thinking about chemical bonding. Web pi bonds are formed from the overlap of parallel p orbitals on adjacent atoms. However, d orbitals can also. They are not formed from hybrid orbitals. Pi bonds are formed by side ways overlap of p orbital which leads lower energy state. From the perspective of quantum mechanics, this bond's weakness is explained by significantly less overlap between the comp… Web the first bond between two atoms is always a sigma bond and the other bonds are always pi. From the perspective of quantum mechanics, this bond's weakness is explained by significantly less overlap between the comp… And a hybridized orbital cannot be involved in a pi bond. Web the three unhybridized 2p orbitals (on c and both o atoms) form three \(\pi\) molecular orbitals, and the remaining 4 electrons occupy both the bonding and nonbonding \(\pi\). Pi bonds. In pi bonds, overlapping takes place at the side of the two. Web pi bonds are formed by sideways overlapping of two parallelly oriented pi orbitals of adjacent atoms. Web the first bond between two atoms is always a sigma bond and the other bonds are always pi bonds. A sigma bond σ is the strongest type of covalent bond. Web the three hybrid orbitals are used to form three sigma bonds, two with the two hydrogen atoms and one with the other carbon atom. The orbitals overlap both above and below the plane of the. As you may see, there is a sideways overlap of the dumbbell shaped p orbitals in the. Due to its parallel structure, it is. Web sigma (σ) and pi (π) bonds form in covalent substances when atomic orbitals overlap. Web the three sp 2 hybrid orbitals lie in one plane, while the unhybridized 2p z orbital is oriented perpendicular to that plane. Illustration of a pi bond forming from two p orbitals. A pi bond is formed. The bonding in c 2 h 4. If we have p orbital in adjacent carbons then. Web what orbitals are used to form the bond indicated in the following molecule? They are not formed from hybrid orbitals. Neither atomic orbitals nor molecular orbitals really exist so they. And a hybridized orbital cannot be involved in a pi bond. Web pi bonds are formed from the overlap of parallel p orbitals on adjacent atoms. Web the three hybrid orbitals are used to form three sigma bonds, two with the two hydrogen atoms and one with the other carbon atom. Web what is the lewis structure of so2? The orbitals overlap both above and below the plane of the. Web. As you may see, there is a sideways overlap of the dumbbell shaped p orbitals in the. Web the three hybrid orbitals are used to form three sigma bonds, two with the two hydrogen atoms and one with the other carbon atom. Ad browse & discover thousands of science book titles, for less. Due to its parallel structure, it is. Web atomic orbitals forming molecular orbitals are a way of thinking about chemical bonding. Web the three hybrid orbitals are used to form three sigma bonds, two with the two hydrogen atoms and one with the other carbon atom. Web sigma (σ) and pi (π) bonds form in covalent substances when atomic orbitals overlap. And a hybridized orbital cannot be. Web the three hybrid orbitals are used to form three sigma bonds, two with the two hydrogen atoms and one with the other carbon atom. As you may see, there is a sideways overlap of the dumbbell shaped p orbitals in the. The orbitals overlap both above and below the plane of the. The best videos and questions to learn. Web the three sp 2 hybrid orbitals lie in one plane, while the unhybridized 2p z orbital is oriented perpendicular to that plane. Web what orbitals are used to form the bond indicated in the following molecule? Web the first bond between two atoms is always a sigma bond and the other bonds are always pi bonds. Neither atomic orbitals nor molecular orbitals really exist so they. Pi bonds are usually weaker than sigma bonds. The best videos and questions to learn about pi bonds. In pi bonds, overlapping takes place at the side of the two. However, d orbitals can also. The most common types are formed by the overlap of p orbitals (fig. As you may see, there is a sideways overlap of the dumbbell shaped p orbitals in the. Web sigma (σ) and pi (π) bonds form in covalent substances when atomic orbitals overlap. Web what is the lewis structure of so2? Web pi bonds are formed by sideways overlapping of two parallelly oriented pi orbitals of adjacent atoms. And a hybridized orbital cannot be involved in a pi bond. They are not formed from hybrid orbitals. Illustration of a pi bond forming from two p orbitals. Pi bonds are formed by side ways overlap of p orbital which leads lower energy state. The bonding in c 2 h 4 is explained as. Ethene, sp2 hybridization with a pi bond. Web pi bonds \((\pi)\) are a type of covalent bond formed by sideways or lateral overlapping of atomic orbitals.Sigma and Pi Bonds Brilliant Math & Science Wiki
Biochemistry Glossary Bonds 3. Pi Bonds Overlap Draw It to Know It
PPT Chapter 2 Structure and Properties of Organic Molecules
How many pi bonds can the atom form? Socratic
Sigma and Pi Bonds — Definition & Overview Expii
[Solved] sketch sigma and pi bond from p orbital Course Hero
PPT Chapter 8 Lecture 8.5 Hybridization PowerPoint Presentation, free
Public Domain Picture Spacefilling model of a pi bond (bonding
Biochemistry Glossary Bonds 2. Pi Bonds Visualization Draw It to
PPT Sigma and Pi Bonding PowerPoint Presentation, free download ID
Related Post: