What Ion Is Iodine Most Likely To Form
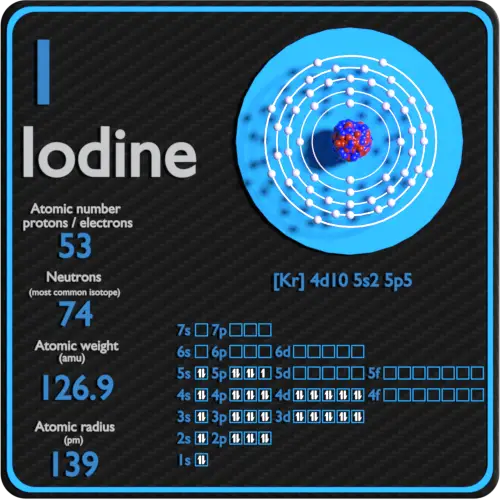
What Ion Is Iodine Most Likely To Form - Web during the formation of some compounds, atoms gain or lose electrons, and form electrically charged particles called ions (figure 3.5.1 ). The iodine atom has an electron configuration of [kr] 4d 10 5s 2 5p 5, or 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 5s 2 5p 5. Web study with quizlet and memorize flashcards containing terms like iodine and potassium form an ionic bond. Understand how do cations form, when ions are formed, how does an atom becomes anion, and do cations. Which of the following would have to. What is the ionic charge when iodine forms an ion? Web the first ionization potential of the iodine atom is considerably smaller than that of the lighter halogen atoms, and this is in accord with the existence of numerous compounds. Iodine has seven electrons in its outer shell, and potassium has one. Thus it is reduced and forms. Web of course we know that iodine is a diatomic molecule, as are all the halogens, and we would write the reduction reaction as. Web what ion is iodine most likely to form? What is the ionic charge when iodine forms an ion? Web based on the octet rule, iodine most likely forms a ion. Compounds between metal and nonmetal elements are usually ionic. You can use the periodic. Web iodine has an electronegativity of 2.66. An element can exist as an ion in. Web how are ions formed? Learn about this topic in these articles: Although the iodide ion is colourless,. You can use the periodic. Thus it is reduced and forms. For example, cabr2 cabr 2 contains a metallic element (calcium, a group 2 [or 2a]. Web study with quizlet and memorize flashcards containing terms like iodine and potassium form an ionic bond. Web the first ionization potential of the iodine atom is considerably smaller than that of the lighter. You can use the periodic. Web of course we know that iodine is a diatomic molecule, as are all the halogens, and we would write the reduction reaction as. Iodine has seven electrons in its outer shell, and potassium has one. Iodine has a melting point of 113.5°c, a boiling point of 184.35°c, a specific gravity of 4.93 for its. Web iodine has an electronegativity of 2.66. Is this a cation or an anion? Which of the following would have to. Based on their locations in the periodic table, which two elements are. What is the ionic charge when iodine forms an ion? Which of the following would have to. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos and images. Web the first ionization potential of the iodine atom is considerably smaller than that of the lighter halogen atoms, and this is in accord with the existence of numerous compounds. Iodine has seven electrons in its outer shell, and potassium has one.. Web based on the octet rule, iodine most likely forms a ion. 1 2 i 2 + e− → i −. Compounds between metal and nonmetal elements are usually ionic. You can use the periodic. The sodium atom transfers electrons to the chlorine atoms to form ionic bonds. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos and images. Web the first ionization potential of the iodine atom is considerably smaller than that of the lighter halogen atoms, and this is in accord with the existence of numerous compounds. Which of the following would have to. Based on their locations in the periodic table, which two elements are.. Web during the formation of some compounds, atoms gain or lose electrons, and form electrically charged particles called ions (figure 3.5.1 ). Based on their locations in the periodic table, which two elements are. Thus it is reduced and forms. An element can exist as an ion in. Web based on the octet rule, iodine most likely forms a ion. Web how are ions formed? What is the ionic charge when iodine forms an ion? Web the first ionization potential of the iodine atom is considerably smaller than that of the lighter halogen atoms, and this is in accord with the existence of numerous compounds. Web based on the octet rule, iodine most likely forms an ________ ion. .iodine thus. Web what ion is iodine most likely to form? Based on their locations in the periodic table, which two elements are. Learn about this topic in these articles: Web based on the octet rule, iodine most likely forms a ion. Iodine has a melting point of 113.5°c, a boiling point of 184.35°c, a specific gravity of 4.93 for its solid state at 20°c, a gas density of 11.27 g/l, with a. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos and images. Compounds between metal and nonmetal elements are usually ionic. Web based on the octet rule, iodine most likely forms an ________ ion. Thus it is reduced and forms. What is the ionic charge when iodine forms an ion? Of course the given electronic configuration reflects the old octet rule. You can use the periodic. Web iodine has an electronegativity of 2.66. Web of course we know that iodine is a diatomic molecule, as are all the halogens, and we would write the reduction reaction as. Which of the following would have to. Web during the formation of some compounds, atoms gain or lose electrons, and form electrically charged particles called ions (figure 3.5.1 ). 1)based on the octet rule, iodine most likely forms an __________ ion. Web the first ionization potential of the iodine atom is considerably smaller than that of the lighter halogen atoms, and this is in accord with the existence of numerous compounds. What is the ionic charge when iodine forms an ion? 1 2 i 2 + e− → i −.Iodine Periodic Table and Atomic Properties
Iodine Facts, Symbol, Discovery, Properties, Uses
What you should know about iodine RobbieMack
Ionic Chemical Bonds Educational Resources K12 Learning, Chemistry
Iodine Facts, Symbol, Discovery, Properties, Uses
How to Find the Ionic Charge for Iodine (I) YouTube
Iodine Chemical Properties, Uses & Applications Britannica
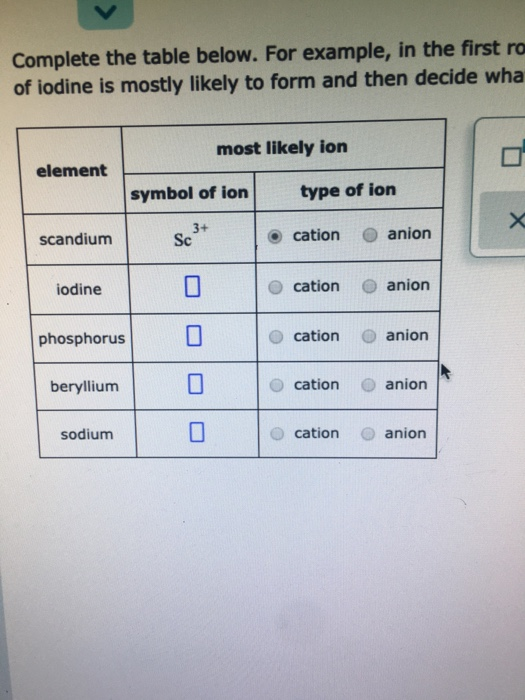
Solved Complete the table below. For example, in the first
Lewis Structures of Ions Mr Pauller YouTube
Iodine, atomic structure Stock Image C018/3734 Science Photo Library
Related Post: