What Ion Does Magnesium Form
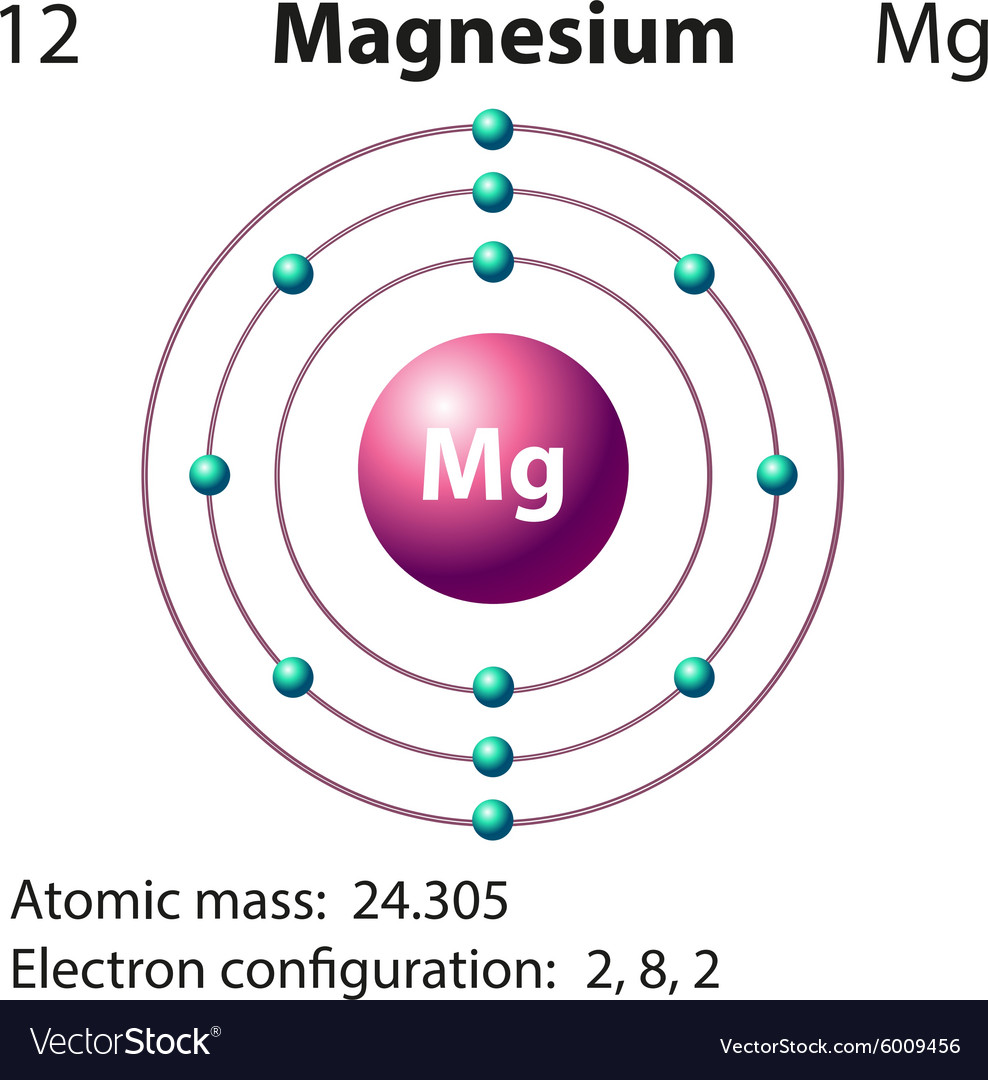
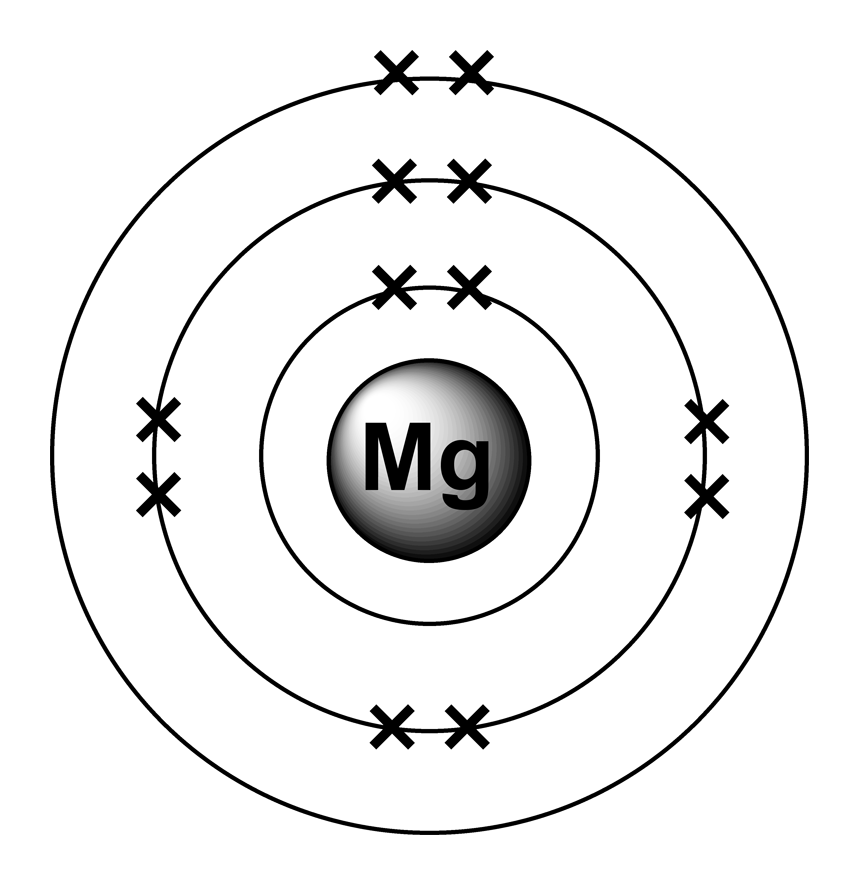
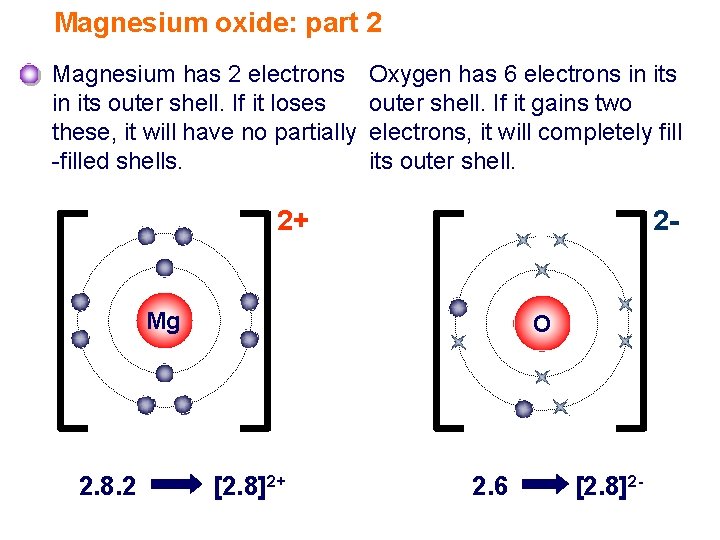
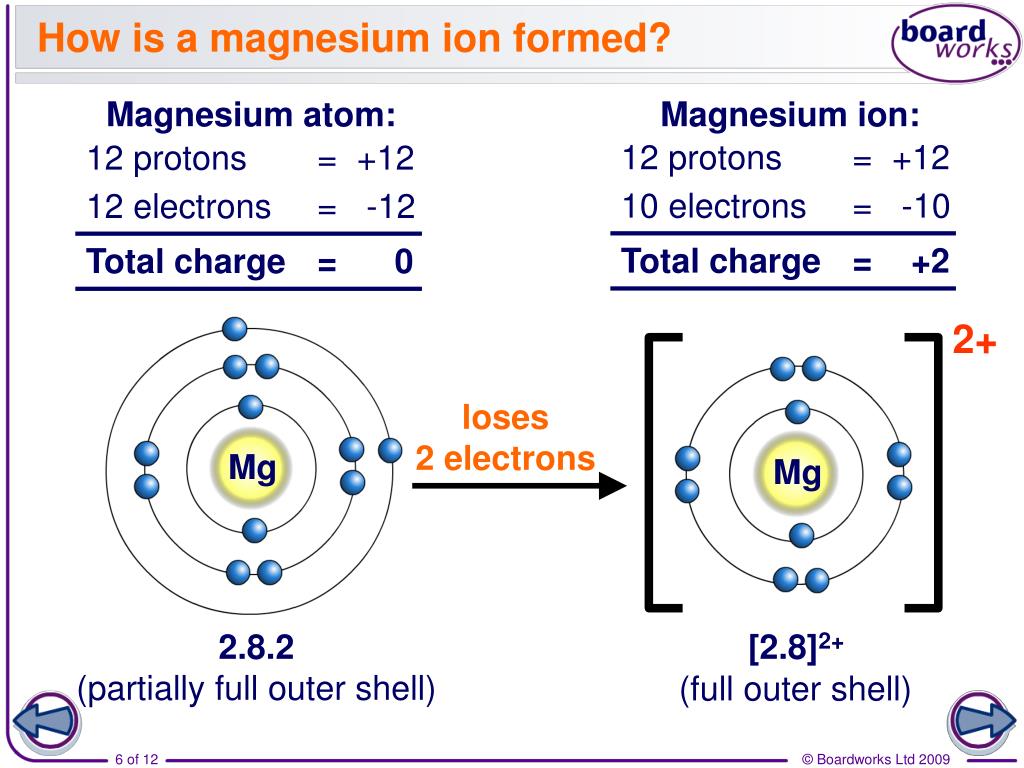
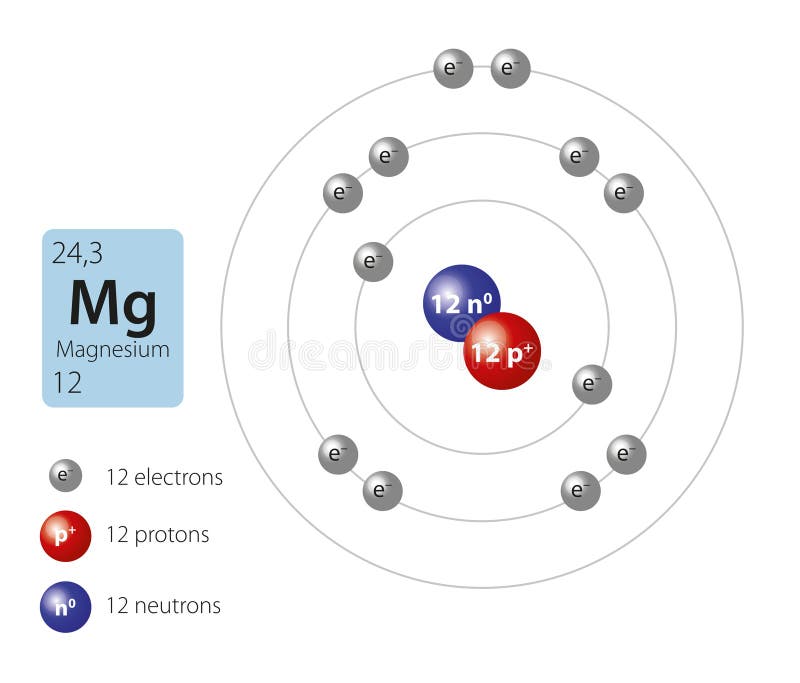
What Ion Does Magnesium Form - When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). Web thus, a magnesium atom will form a cation with two fewer electrons than protons and a charge of 2+. Because it usually forms cations of only one type, we don't need to specify its charge. Like the other alkaline earth metals (group 2 of the periodic table) it occurs naturally only in combination with other elements and it almost always has an oxidation state of +2. Nonmetals form negative ions (anions). Magnesium is required for energy production, oxidative phosphorylation, and glycolysis. Web in plants, magnesium is the central ion of chlorophyll [ 3 ]. Web to balance the charges with the lowest number of ions possible, we need to have two chloride ions to balance the charge on the one magnesium ion. Web magnesium is an alkaline earth metal. It naturally forms a white, powdery substance and may be sold in powder or capsule form ().this type isn’t typically used to prevent. Rather than write the formula mgclcl, we combine the two chloride ions and write it with a 2 subscript: Because it usually forms cations of only one type, we don't need to specify its charge. This electric charge generated on. Dark, leafy greens such as spinach. Web to balance the charges with the lowest number of ions possible, we need. Rather than write the formula mgclcl, we combine the two chloride ions and write it with a 2 subscript: Web a magnesium atom gives two electrons to two chlorine atoms to form a magnesium ion and two chloride ions. For example, adenosine triphosphate (atp), the main source of energy in cells, must bind to a magnesium ion in order to. The magnesium ion and the carbonate ion; Web next generation and beyond lithium chemistries. For example, adenosine triphosphate (atp), the main source of energy in cells, must bind to a magnesium ion in order to be biologically active. This electric charge generated on. It combines easily with oxygen and at high temperatures reacts with such nonmetals as the halogens, sulfur,. Web next generation and beyond lithium chemistries. Computed by pubchem 2.1 (pubchem release 2021.05.07) element name. The charge on a magnesium ion is +2. Web boost vitamin d absorption. Magnesium is required for energy production, oxidative phosphorylation, and glycolysis. Like the other alkaline earth metals (group 2 of the periodic table) it occurs naturally only in combination with other elements and it almost always has an oxidation state of +2. Seeds, such as pumpkin or chia. It is an essential mineral nutrient (i.e., element) for life [1] [2] [3] [4] and is present in every cell type in every. The aluminum ion and the acetate ion; When reacted with bases, magnesium react. Web next generation and beyond lithium chemistries. The charge on a magnesium ion is +2. Many enzymes depend on magnesium for their functioning. Web in plants, magnesium is the central ion of chlorophyll [ 3 ]. Magnesium is an element that is essential for human nutrition. All elements can and many often do. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos and images. Web magnesium oxide is a salt that combines magnesium and oxygen. It combines easily with oxygen and at high temperatures reacts with such nonmetals as the halogens, sulfur, and even nitrogen. Web write the chemical formula for an ionic compound composed of each pair of ions. Nonmetals form negative ions (anions). Web what does the charge on the magnesium ion have to do with the number of valence electrons that an. Web magnesium, mg , is a group 2 element that will form 2+ cations. Magnesium is required for energy production, oxidative phosphorylation, and glycolysis. It is a shiny gray metal having a low density, low melting point and high chemical reactivity. Nuts, such as almonds and cashews. Nonmetals form negative ions (anions). Chorine is in the seventh column and therefore has 7 electrons in its outermost shell. Like the other alkaline earth metals (group 2 of the periodic table) it occurs naturally only in combination with other elements and it almost always has an oxidation state of +2. We can simply refer to the cation in the ionic compound as magnesium. This. The charge on a magnesium ion is +2. Web next generation and beyond lithium chemistries. Rather than write the formula mgclcl, we combine the two chloride ions and write it with a 2 subscript: Beans, such as black beans. Because it usually forms cations of only one type, we don't need to specify its charge. Web magnesium is an alkaline earth metal. Web what does the charge on the magnesium ion have to do with the number of valence electrons that an atom of magnesium has? Nitrogen’s position in the periodic table (group 15) reveals that it is a nonmetal. Nuts, such as almonds and cashews. Nonmetals form negative ions (anions). Web magnesium oxide is a salt that combines magnesium and oxygen. Computed by pubchem 2.1 (pubchem release 2021.05.07) element name. When reacted with acids, magnesium dissolves and forms solutions that have both the mg(ii) ion and hydrogen gas. Like the other alkaline earth metals (group 2 of the periodic table) it occurs naturally only in combination with other elements and it almost always has an oxidation state of +2. Since these ions, lithium or magnesium, are effectively shuttling the electrons back and forth the magnesium. Web boost vitamin d absorption. Web magnesium is essential to all living cells, as the mg 2+ ion is involved with the critically important biological polyphosphate compounds dna, rna, and adenosine triphosphate (atp). We can simply refer to the cation in the ionic compound as magnesium. It is an essential mineral nutrient (i.e., element) for life [1] [2] [3] [4] and is present in every cell type in every organism. All elements can and many often do.Diagram representation of the element magnesium Vector Image
Electron configurations
Magnesium, atomic structure Stock Image C018/3693 Science Photo
Ionic Bonding Elements are the simplest substances There
Magnesium Mg (Elements 12) of Periodic Table Elements FlashCards
PPT How do atoms form ions? PowerPoint Presentation, free download
Magnesium Description, Properties, & Compounds Britannica
Model of magnesium atom stock vector. Illustration of mass 164475021
EXPLAIN WHY MAGNESIUM FORMS MG ION Brainly.in
Periodic Table Magnesium Electron Configuration Periodic Table Timeline
Related Post:




/GettyImages-1135707671-640473b29d534e15a24491c0d6b2789e.jpg)