What Bonds Form Between Amino Acids
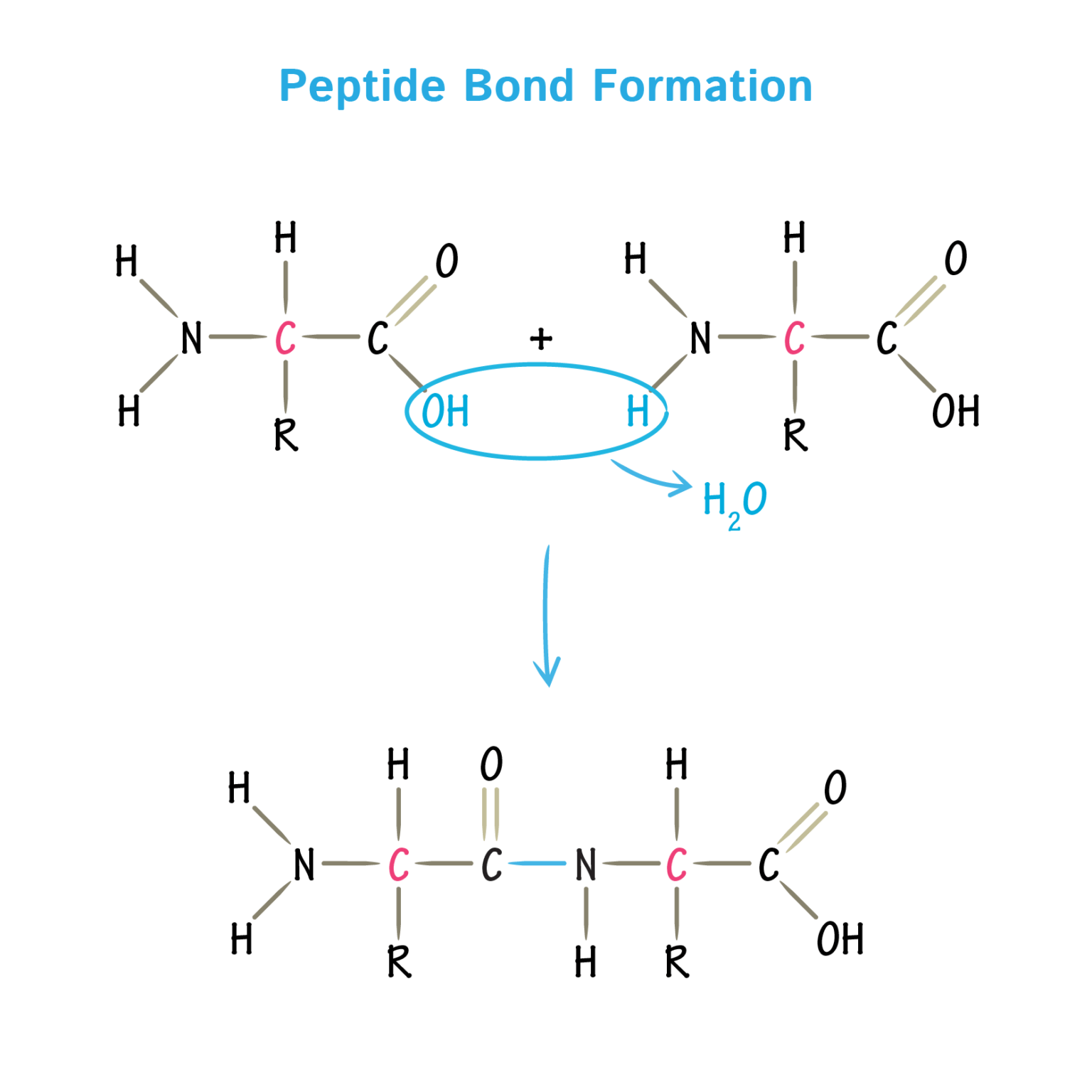
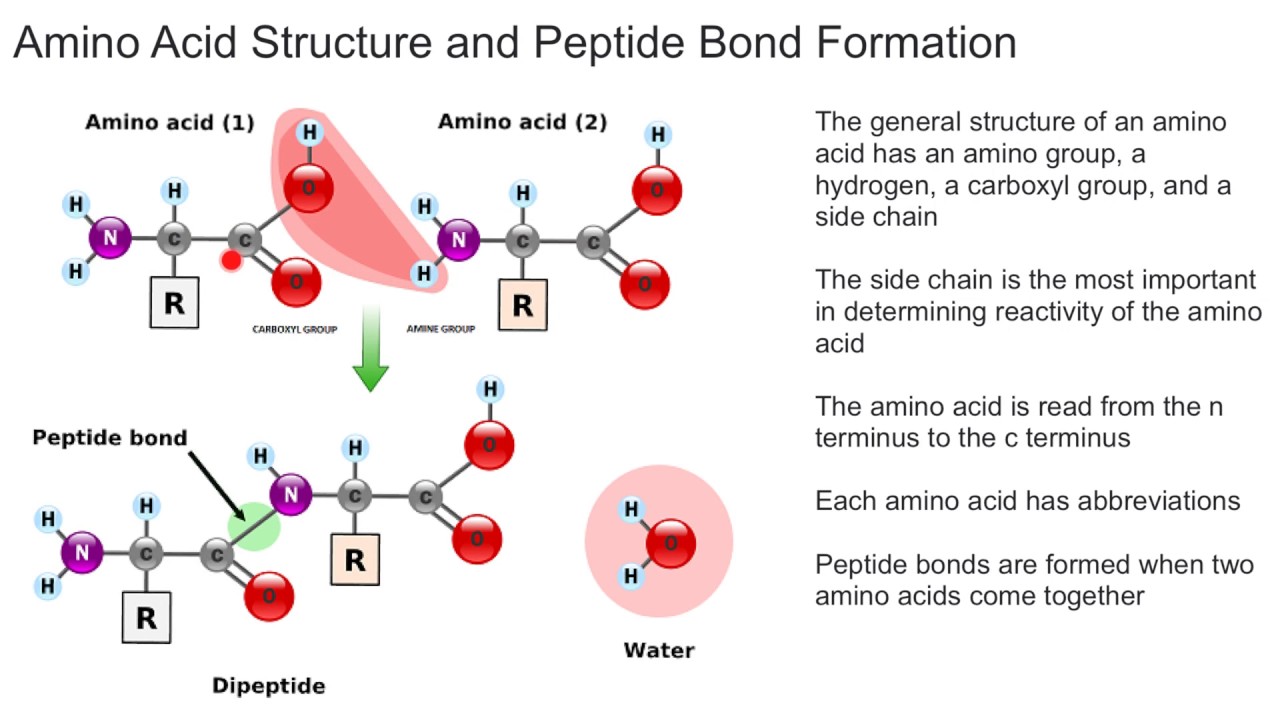
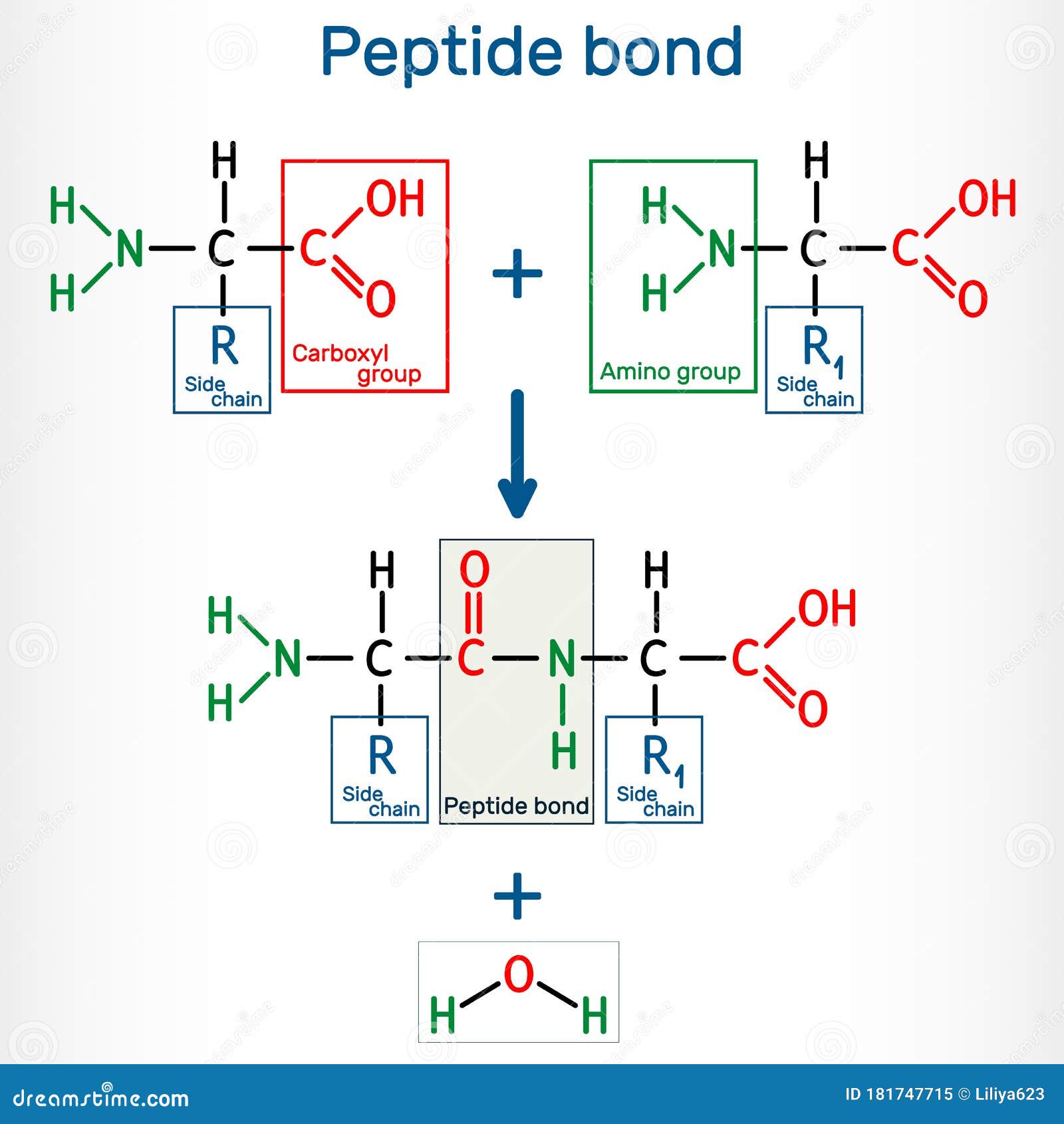
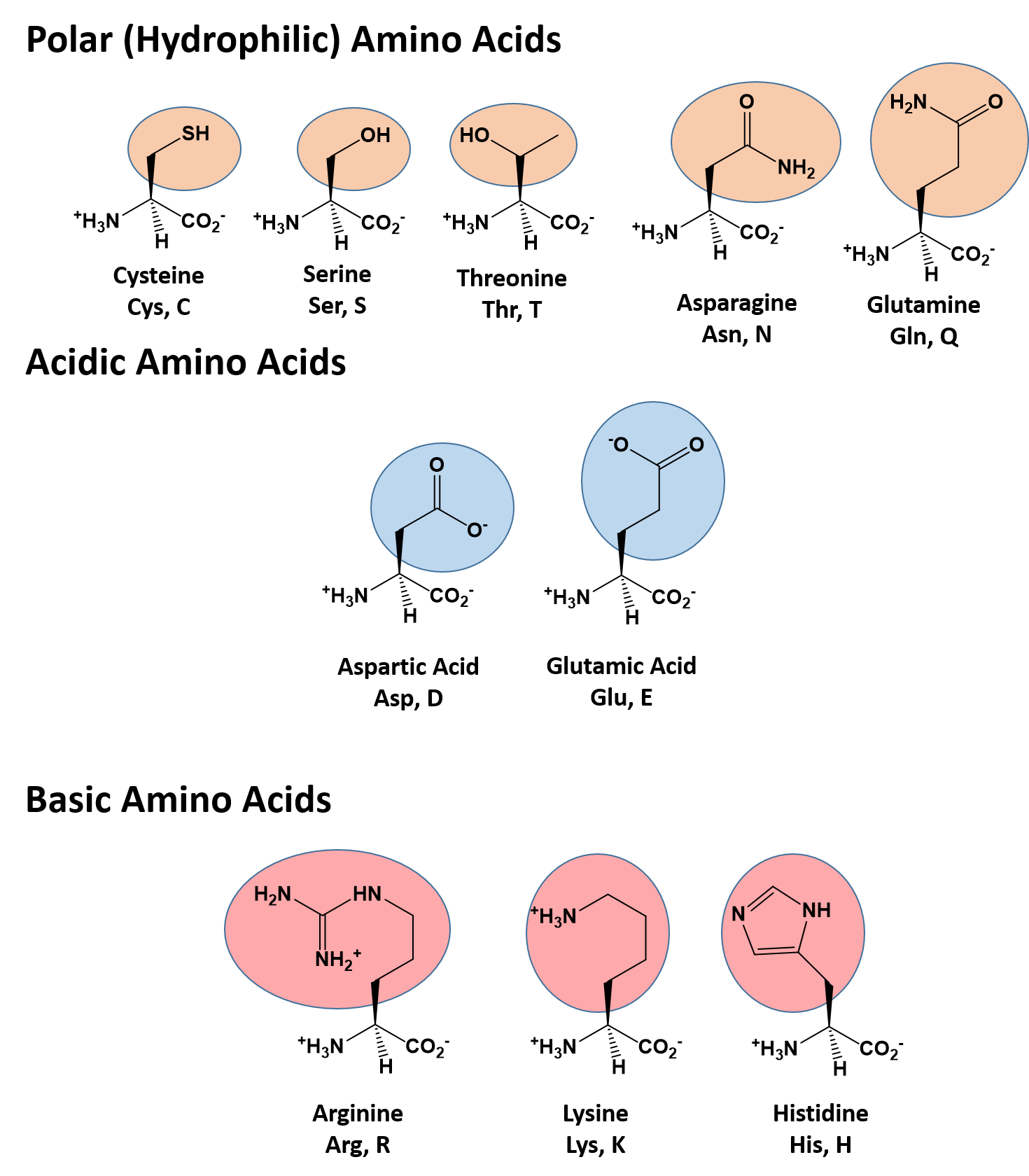
What Bonds Form Between Amino Acids - Web the amino group of one amino acid can react with the carboxyl group on another amino acid to form a peptide bond that links the two amino acids together. The forces in secondary structure primarily involve hydrogen bonds. Image modified from openstax biology. Web now let's turn our attention to the way in which amino acids are linked together to form proteins. At the turn of the 20th century, german chemist. Thus, ionization/ deionization in a protein arises only from 1) the amino terminus; The peptide bond is the bond between the carbon atom and the nitrogen atom. This bond is important for life because when linking. Web the bond that holds together the two amino acids is a peptide bond, or a covalent bond.it occurs when the carboxylic group of one molecule reacts with the amino group of the other molecule, linking the two molecules and releasing a water molecule. Images showing hydrogen bonding patterns in beta pleated sheets and alpha helices. Additional amino acids can be added on through the formation of. This bond is important for life because when linking. Propagation a polar reaction initiation termination Web peptide bonds are chemical covalent bonds linking one amino acid to the other, and they form between a carbon atom of one amino acid and a nitrogen atom of the other amino. This. These naturally occurring amino acids are used by cells to synthesize peptides and proteins. The key structural element here is the peptide bond. If so, does this make it an overall energy neutral reaction? Web amino acids are joined by peptide bonds. The primary difference between the 20 amino acids is a different structure of r group. Web amino acids are joined by peptide bonds. Web a peptide bond is a covalent bond formed between two amino acids. A schematical representation of this bond is shown below. Web 1 day agoscience biochemistry proteins are made from chains of amino acids. Web amino acid, any of a group of organic molecules that consist of a basic amino group,. When two amino acids form a dipeptide through a peptide bond, [1] it is a type of condensation reaction. Web to form polypeptides and proteins, amino acids are joined together by peptide bonds, in which the amino or nh 2 of one amino acid bonds to the carboxyl (acid) or cooh group of another amino acid. Image modified from openstax. Web the amino group of one amino acid can react with the carboxyl group on another amino acid to form a peptide bond that links the two amino acids together. Additional amino acids can be added on through the formation of. A polypeptide is a chain of many amino acids; A peptide bond is a type of covalent bond between. Web only 20 amino acids are found in the human peptides and proteins. Web when you form proteins in the ribosomes, is the energy required to form a peptide bond between two amino acids from the breaking of the bond between the amino acid and the trna molecule? Examples of amino acids include glycine and threonine. Web now let's turn. They are typically identified by generic formula: If so, does this make it an overall energy neutral reaction? Web peptide bonds are chemical covalent bonds linking one amino acid to the other, and they form between a carbon atom of one amino acid and a nitrogen atom of the other amino. Images showing hydrogen bonding patterns in beta pleated sheets. Image modified from openstax biology. Web charged amino acid side chains can form ionic bonds, and polar amino acids are capable of forming hydrogen bonds. Step 1 is an example of: Peptide bonds between amino acids give rise to which feature of protein structure? Examples of amino acids include glycine and threonine. Thus, ionization/ deionization in a protein arises only from 1) the amino terminus; A schematical representation of this bond is shown below. Step 1 is an example of: The primary difference between the 20 amino acids is a different structure of r group. They are typically identified by generic formula: At the turn of the 20th century, german chemist. If so, does this make it an overall energy neutral reaction? Web these include peptide bonds, which link amino acids together in a linear sequence. Images showing hydrogen bonding patterns in beta pleated sheets and alpha helices. Web what is the bond that forms between two amino acids? A schematical representation of this bond is shown below. Image modified from openstax biology. Web each individual amino acid has an amino group (nh 2) and a carboxyl (cooh) group. Polypeptides are formed when the amino group of one amino acid forms an amide (i.e., peptide) bond with the carboxyl group of another amino acid ( figure 15.15 ). Web the bond that holds together the two amino acids is a peptide bond, or a covalent bond.it occurs when the carboxylic group of one molecule reacts with the amino group of the other molecule, linking the two molecules and releasing a water molecule. Web these include peptide bonds, which link amino acids together in a linear sequence. The primary difference between the 20 amino acids is a different structure of r group. Web amino acids are joined by peptide bonds. These naturally occurring amino acids are used by cells to synthesize peptides and proteins. A polypeptide is a chain of many amino acids; Hydrophobic side chains interact with each other via weak van der waals. They are typically identified by generic formula: A peptide bond is a type of covalent bond between the carboxyl group of one amino acid and the amino group of another amino acid. Peptide bonds between amino acids give rise to which feature of protein structure? This reaction is catalyzed by ribosomes and generates one water molecule. The bond between two amino acids is formed under the formation of h_2o. This bond is called a peptide bond (a covalent bond). The forces in secondary structure primarily involve hydrogen bonds. Tertiary structure primary structure quaternary structure secondary structure question 8 step 1 step 2 step 3 step 4 consider the mechanism above. Additional amino acids can be added on through the formation of.Amino acids physical, chemical properties and peptide bond
Amino Acid Structure and Peptide Bond Formation YouTube
How do you Identify a Peptide Bond?
An amino acid has the following structure. Which two group combine to
amino acids salt bridge vs hydrogen bond Chemistry Stack Exchange
USC Bridge 2.5 proteins Flashcards Easy Notecards
Amino Acids Structure Nutrition
Peptide Bond. Formation of Amide Bonds from Two Amino Acids As a Result
CH103 Chapter 8 The Major Macromolecules Chemistry
Two amino acids are joined together by
Related Post: