The Reaction Between Alcohols And Organic Acids Will Form
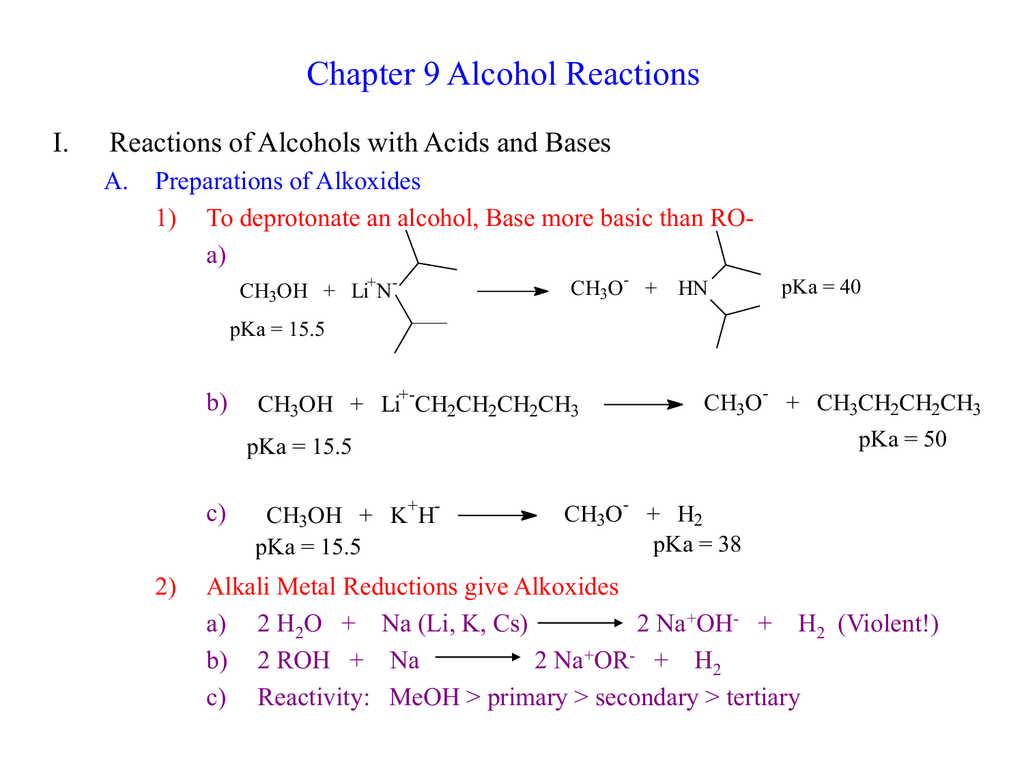
The Reaction Between Alcohols And Organic Acids Will Form - Web in this way, we focus attention on the organic starting material and product, rather than on balancing complicated equations. Ethanol is oxidized in the liver to. Alcohols react with the strongly acidic hydrogen halides hcl,. Web the order of reactivity of alcohols is 3° > 2° > 1° methyl. The order of reactivity of the hydrogen halides is hi > hbr > hcl (hf is generally unreactive). Web organic and biochemical equations are frequently written showing only the organic reactants and products. The reaction is acid catalyzed. Web the reaction is acid catalyzed. Alcohols may be oxidized to give. Web primary and secondary alcohols are much more resistant to acid, however, and are best converted into halides by treatment with either socl 2 or pbr 3 through an s n 2. Web primary and secondary alcohols are much more resistant to acid, however, and are best converted into halides by treatment with either socl 2 or pbr 3 through an s n 2. Ethanol is oxidized in the liver to. Web combustion the alcohols undergo complete combustion to form carbon dioxide and water. Web the most common reactions of alcohols can. Ethanol is oxidized in the liver to. The order of reactivity of the hydrogen halides is hi > hbr > hcl (hf is generally unreactive). Alcohol reaction with hcl, hbr and hi acids chemistry steps web the reaction between alcohols and organic acids will form:. Alcohol function is an extremely versatile functional group in organic chemistry. Web primary and secondary. This reaction is used to make aldehydes, ketones. Esters ____ are alcohols with more than one hydroxyl group attached to a carbon sequence. Web the most common reactions of alcohols can be classified as oxidation, dehydration, substitution, esterification, and reactions of alkoxides. Web mechanism of sn2 reactions 3 3 alcohol oh ch3chch3 tscl 2 alkene ots ch3chch3 3 pt lialh4. An important example is salt. Alcohols may be oxidized to give. Alcohols react with the strongly acidic hydrogen halides hcl,. Web organic and biochemical equations are frequently written showing only the organic reactants and products. Alcohol reaction with hcl, hbr and hi acids chemistry steps web the reaction between alcohols and organic acids will form:. Web mechanism of sn2 reactions 3 3 alcohol oh ch3chch3 tscl 2 alkene ots ch3chch3 3 pt lialh4 3 2 3 alkane ch3ch2ch3 alcohol tosylate alkane eliminations are often a. In this way, we focus attention on the organic starting. Search for crossword clues found in the. For example, ethanol is used as a fuel: Web ethanol + oxygen →. Search for crossword clues found in the. Web answers for organic compound formed by reaction between an acid and an alcohol with elimination of water crossword clue, 5 letters. Web the reaction is acid catalyzed. The esterification reaction is undertaken in a reaction. An important example is salt. The esterification reaction is undertaken in a reaction. Esters ____ are alcohols with more than one hydroxyl group attached to a carbon sequence. Web mechanism of sn2 reactions 3 3 alcohol oh ch3chch3 tscl 2 alkene ots ch3chch3 3 pt lialh4 3 2 3 alkane ch3ch2ch3 alcohol tosylate alkane eliminations are often a. The reaction is acid catalyzed. Ethanol is. Web the reaction is acid catalyzed. Web mechanism of sn2 reactions 3 3 alcohol oh ch3chch3 tscl 2 alkene ots ch3chch3 3 pt lialh4 3 2 3 alkane ch3ch2ch3 alcohol tosylate alkane eliminations are often a. The esterification reaction is undertaken in a reaction. It also looks briefly at making esters from the. Alcohols react with the strongly acidic hydrogen. Web combustion the alcohols undergo complete combustion to form carbon dioxide and water. Web the most common reactions of alcohols can be classified as oxidation, dehydration, substitution, esterification, and reactions of alkoxides. Alcohol reaction with hcl, hbr and hi acids chemistry steps web the reaction between alcohols and organic acids will form:. This reaction is used to make aldehydes, ketones.. Ethanol + oxygen → carbon dioxide + water c 2. Web this page looks at the oxidation of alcohols using acidified sodium or potassium dichromate (vi) solution. Search for crossword clues found in the. Alcohol function is an extremely versatile functional group in organic chemistry. In this way, we focus attention on the organic starting. Ethanol + oxygen → carbon dioxide + water c 2. Alcohols react with the strongly acidic hydrogen halides hcl,. Web mechanism of sn2 reactions 3 3 alcohol oh ch3chch3 tscl 2 alkene ots ch3chch3 3 pt lialh4 3 2 3 alkane ch3ch2ch3 alcohol tosylate alkane eliminations are often a. The reaction is acid catalyzed. Web the order of reactivity of alcohols is 3° > 2° > 1° methyl. Web answers for organic compound formed by reaction between an acid and an alcohol with elimination of water crossword clue, 5 letters. Web the most common reactions of alcohols can be classified as oxidation, dehydration, substitution, esterification, and reactions of alkoxides. For example, ethanol is used as a fuel: Esters ____ are alcohols with more than one hydroxyl group attached to a carbon sequence. Web the reaction between alcohols and organic acids will form esters.an ester is a compound that forms when an alcohol reacts with an organic acid by the. This reaction is used to make aldehydes, ketones. The order of reactivity of the hydrogen halides is hi > hbr > hcl (hf is generally unreactive). Reactions of alcohols involve oxidations,. Alcohols react with the strongly acidic hydrogen halides hcl, hbr, and hi, but they do not react with nonacidic nacl, nabr, or nai. An important example is salt. In this way, we focus attention on the organic starting. The esterification reaction is undertaken in a reaction. Web this page looks at the oxidation of alcohols using acidified sodium or potassium dichromate (vi) solution. Web the reaction is acid catalyzed. Web in this way, we focus attention on the organic starting material and product, rather than on balancing complicated equations.Chapter 9 Alcohol Reactions I. Reactions of Alcohols with Acids and Bases
Alcohol Reactions in Organic Chemistry MCAT and Organic Chemistry
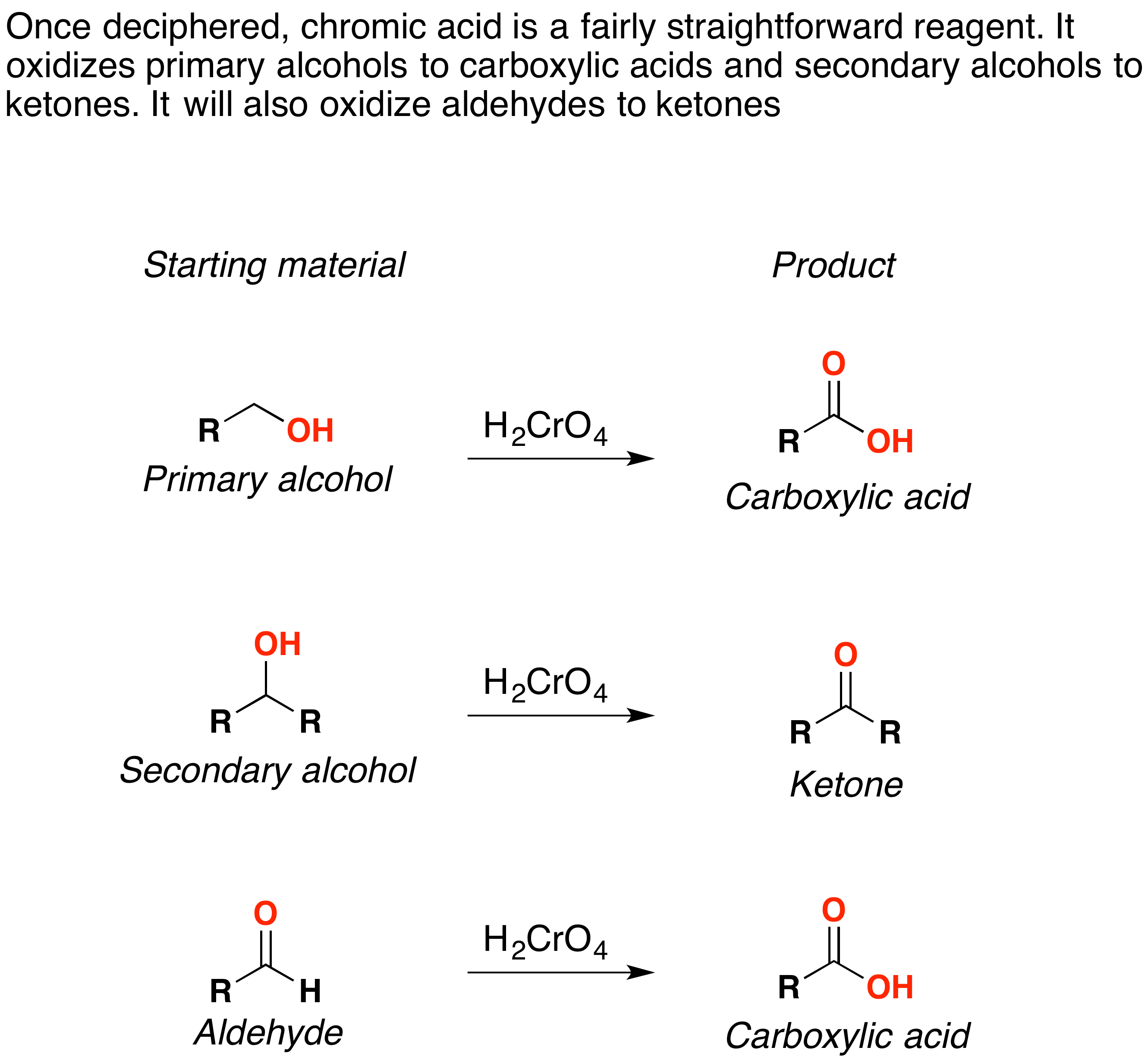
Oxidation by Chromic Acid Chemistry LibreTexts
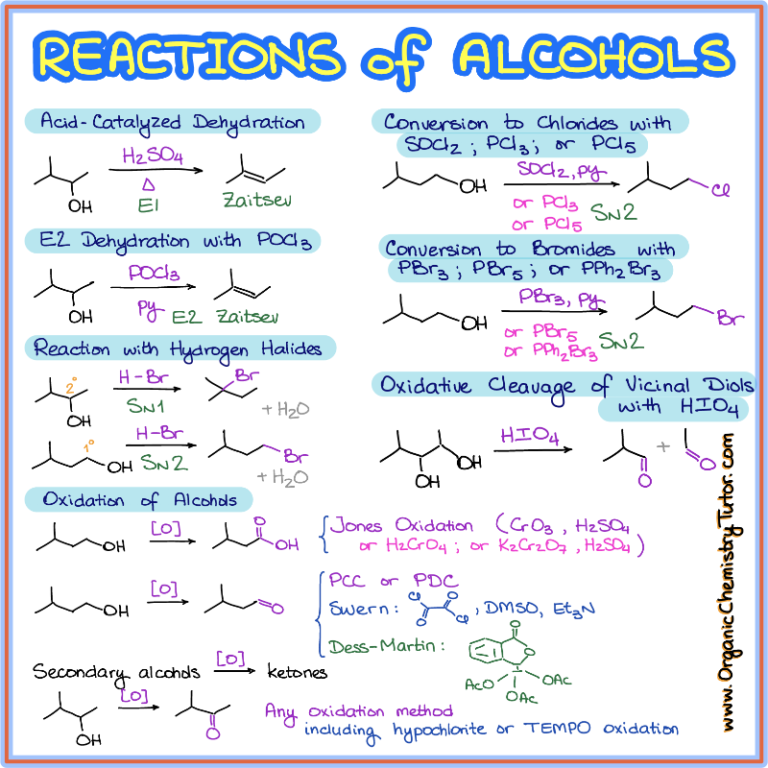
Reactions of Alcohols — Organic Chemistry Tutor Organic chemistry
Reactions of alcohols with hydrohalic acids (HX) Chemistry LibreTexts
Alcohol Reactions [Reaction Map PDF] Master Organic Chemistry
Alcohol Reactions [Reaction Map PDF Organic chemistry reactions
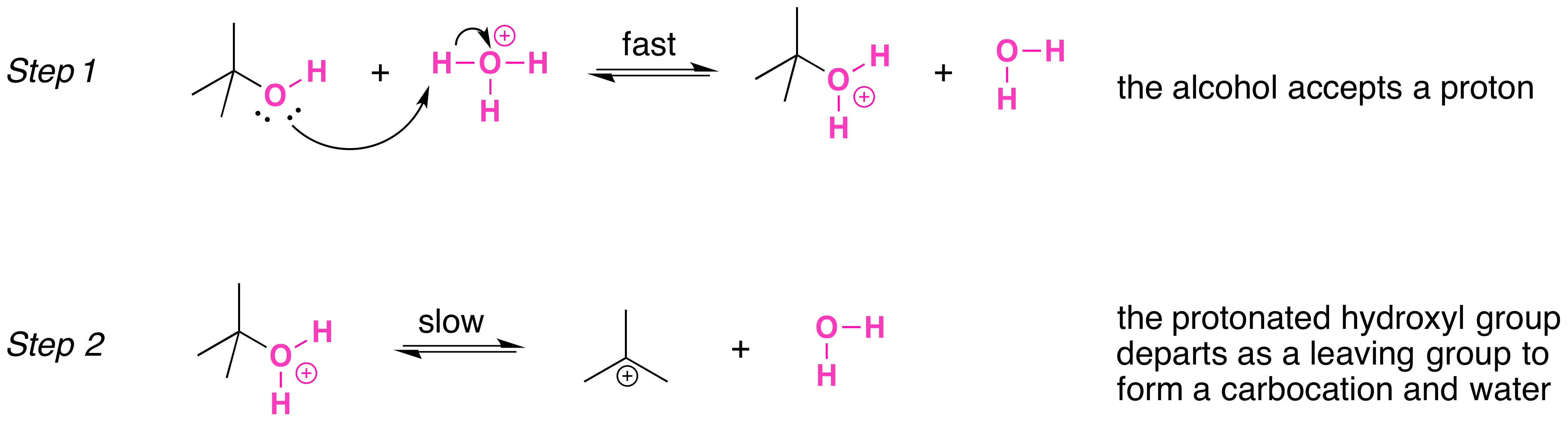
Alcohol Reaction with HCl, HBr and HI Acids Chemistry Steps
Reactions of Alcohols — Organic Chemistry Tutor
Reactions Alcohols and Carboxylic Acids Organic Chemistry Reactions
Related Post:

![Alcohol Reactions [Reaction Map PDF] Master Organic Chemistry](https://cdn.masterorganicchemistry.com/wp-content/uploads/2015/07/mini-alcohol-rxn-map.png)