Sulfur Electron Configuration Long Form
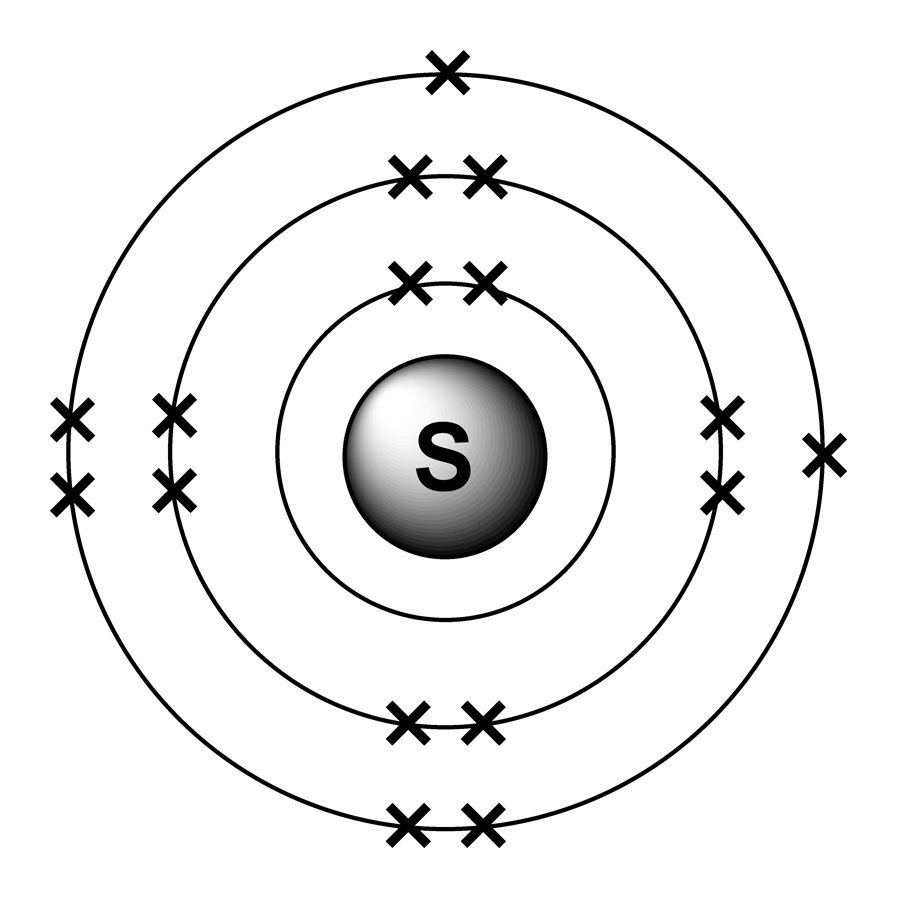
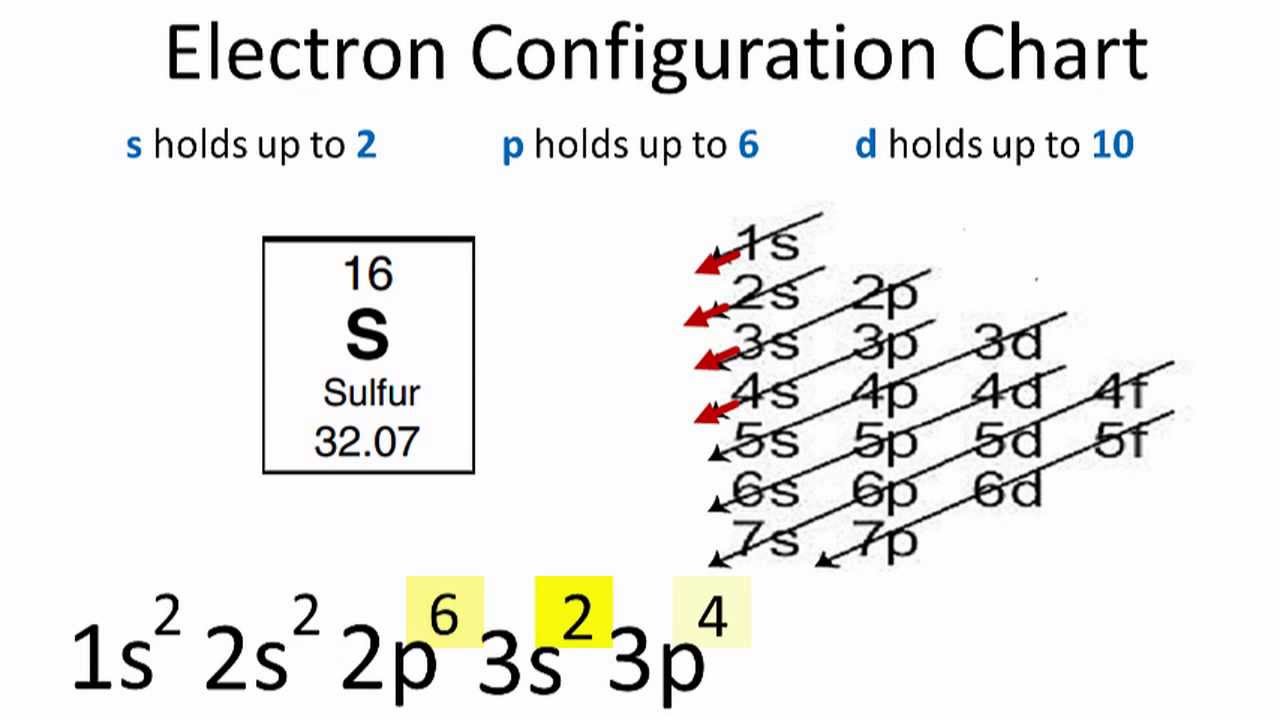
Sulfur Electron Configuration Long Form - Sulfur polycations, s8 , s4 and s16 are produced when sulfur is reacted with oxidising agents in a strongly acidic solution. For each atom the subshells are given first in concise form, then with all. Sulfur is a beautiful yellow crystalline solid at room temperature. Sulfur have 6 valence electrons around the nucleus and the atomic number is 16.the. The first is more conceptually cohesive and involves using the periodic table to write the notation. Web it is nonmetallic, abundant, and multivalent. In this article, we will study how electrons are arranged in different shells and subshells of sulphur. Electron configuration the periodic table is a tabular display of the. 1s 2 2s 2 2p 1: Web electron configuration the arrangements of electrons above the last (closed shell) noble gas. Web electron configuration of sulfur is [ne] 3s2 3p4. Web when we write the configuration we'll put all 16electrons in orbitals around the nucleus of the sulfur atom. Web there are two ways to write the electronic configuration of electrons in atoms. Common oxidation states of sulfur range from −2 to +6. Sulfur is a beautiful yellow crystalline solid at. Web the atomic number of sulfur represents the total number of electrons of sulfur. For each atom the subshells are given first in concise form, then with all. 1s 2 2s 2 2p 2: Electron configuration of carbon (c) [he] 2s 2 2p 2: Web the long form electron configuration of sulfur (s), which has an atomic number of 16,. Web the complete electron configuration of sulfur is written as 1s22s2 2p63s23p4. Web the atomic number of sulfur represents the total number of electrons of sulfur. Web there are two ways to write the electronic configuration of electrons in atoms. 1s 2 2s 2 2p 2: Its atoms combine to produce cyclic octaatomic molecules. Since it is the outermost (valence) electrons which. Web the atomic number of sulfur represents the total number of electrons of sulfur. Web there are two ways to write the electronic configuration of electrons in atoms. Sulfur polycations, s8 , s4 and s16 are produced when sulfur is reacted with oxidising agents in a strongly acidic solution. The p, d,. Web the commonly used long form of the periodic table is designed to emphasize electron configurations. Web electron configuration of boron (b) [he] 2s 2 2p 1: Since it is the outermost (valence) electrons which. Web the complete electron configuration of sulfur is written as 1s22s2 2p63s23p4. In this video we'll use the electron configuration chart to help us write. Sulfur have 6 valence electrons around the nucleus and the atomic number is 16.the. Sulfur is a beautiful yellow crystalline solid at room temperature. Its atoms combine to produce cyclic octaatomic molecules. Web the commonly used long form of the periodic table is designed to emphasize electron configurations. Sulfur forms stable compounds with all elements except the noble gases. Web the long form electron configuration of sulfur (s), which has an atomic number of 16, is 1s² 2s² 2p⁶ 3s² 3p⁴. Web electron configuration the arrangements of electrons above the last (closed shell) noble gas. Its atoms combine to produce cyclic octaatomic molecules. Electron configuration the periodic table is a tabular display of the. Sulfur have 6 valence electrons. Web the complete electron configuration of sulfur is written as 1s22s2 2p63s23p4. Web when we write the configuration we'll put all 16electrons in orbitals around the nucleus of the sulfur atom. Web sulphur electron configuration is: Electron configuration of carbon (c) [he] 2s 2 2p 2: Since the atomic number of sulfur is 16, the total electrons of sulfur are. Since it is the outermost (valence) electrons which. Since the atomic number of sulfur is 16, the total electrons of sulfur are 16. Web when we write the configuration we'll put all 16electrons in orbitals around the nucleus of the sulfur atom. 1s 2 2s 2 2p 1: The p, d, and f orbitals have different sublevels, thus. The colored solutions produced by dissolving sulfur in oleum were first reported as early as 1804 by c.f. Web when we write the configuration we'll put all 16electrons in orbitals around the nucleus of the sulfur atom. In this video we'll use the electron configuration chart to help us write. Since the atomic number of sulfur is 16, the total. Web the complete electron configuration of sulfur is written as 1s22s2 2p63s23p4. The p, d, and f orbitals have different sublevels, thus. Web electron configuration of sulfur is [ne] 3s2 3p4. Web there are two ways to write the electronic configuration of electrons in atoms. Electron configuration of carbon (c) [he] 2s 2 2p 2: In this video we'll use the electron configuration chart to help us write. The colored solutions produced by dissolving sulfur in oleum were first reported as early as 1804 by c.f. 1s 2 2s 2 2p 2: 1s 2 2s 2 2p 1: Web the electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon (18 electrons) are analogous in the. Web this page shows the electron configurations of the neutral gaseous atoms in their ground states. Sulfur have 6 valence electrons around the nucleus and the atomic number is 16.the. Electron configuration the periodic table is a tabular display of the. Web it is nonmetallic, abundant, and multivalent. Common oxidation states of sulfur range from −2 to +6. Web electron configuration the arrangements of electrons above the last (closed shell) noble gas. The first is more conceptually cohesive and involves using the periodic table to write the notation. Web when we write the configuration we'll put all 16electrons in orbitals around the nucleus of the sulfur atom. Web electron configuration of boron (b) [he] 2s 2 2p 1: Web sulfur, nonmetallic chemical element, one of the most reactive of the elements.Chemist atom of sulfur diagram 528624 Vector Art at Vecteezy
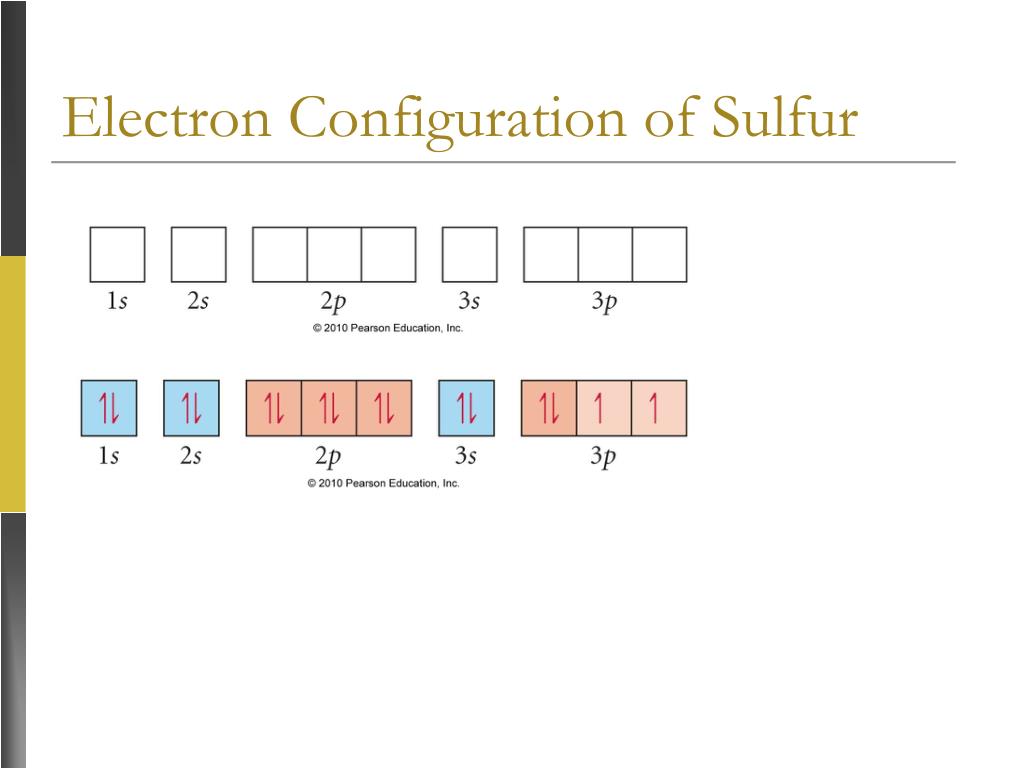
Show The Orbital Filling Diagram For Sulfur
Periodic Table Sulfur Element Periodic Table Timeline
PPT Chapter 6 Electronic Structure of Atoms PowerPoint Presentation
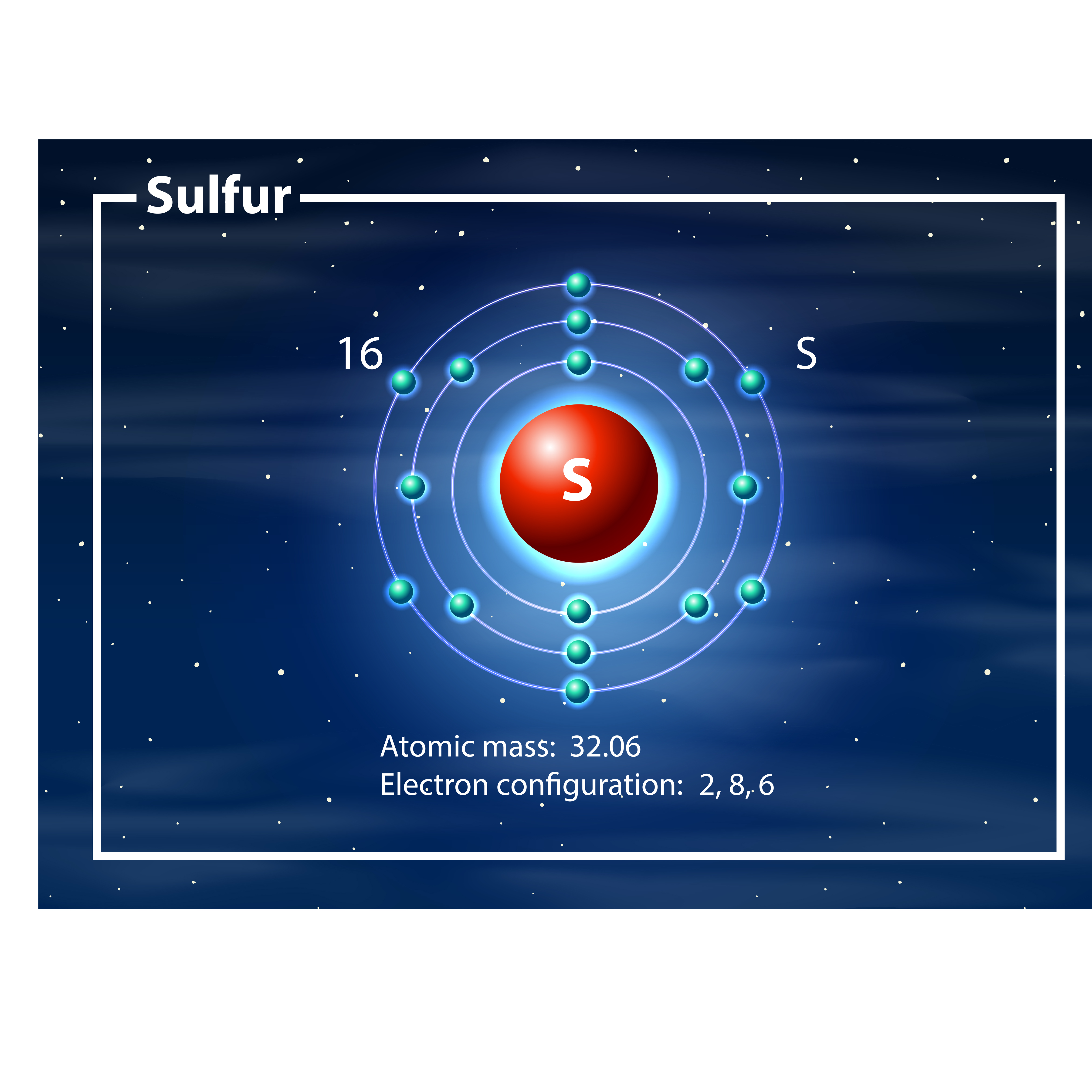
Sulfur Table of Elements by Shrenil Sharma
Sulfur Atom Science Notes and Projects
Sulfur Electron Configuration YouTube
Atom Diagrams Electron Configurations of the Elements
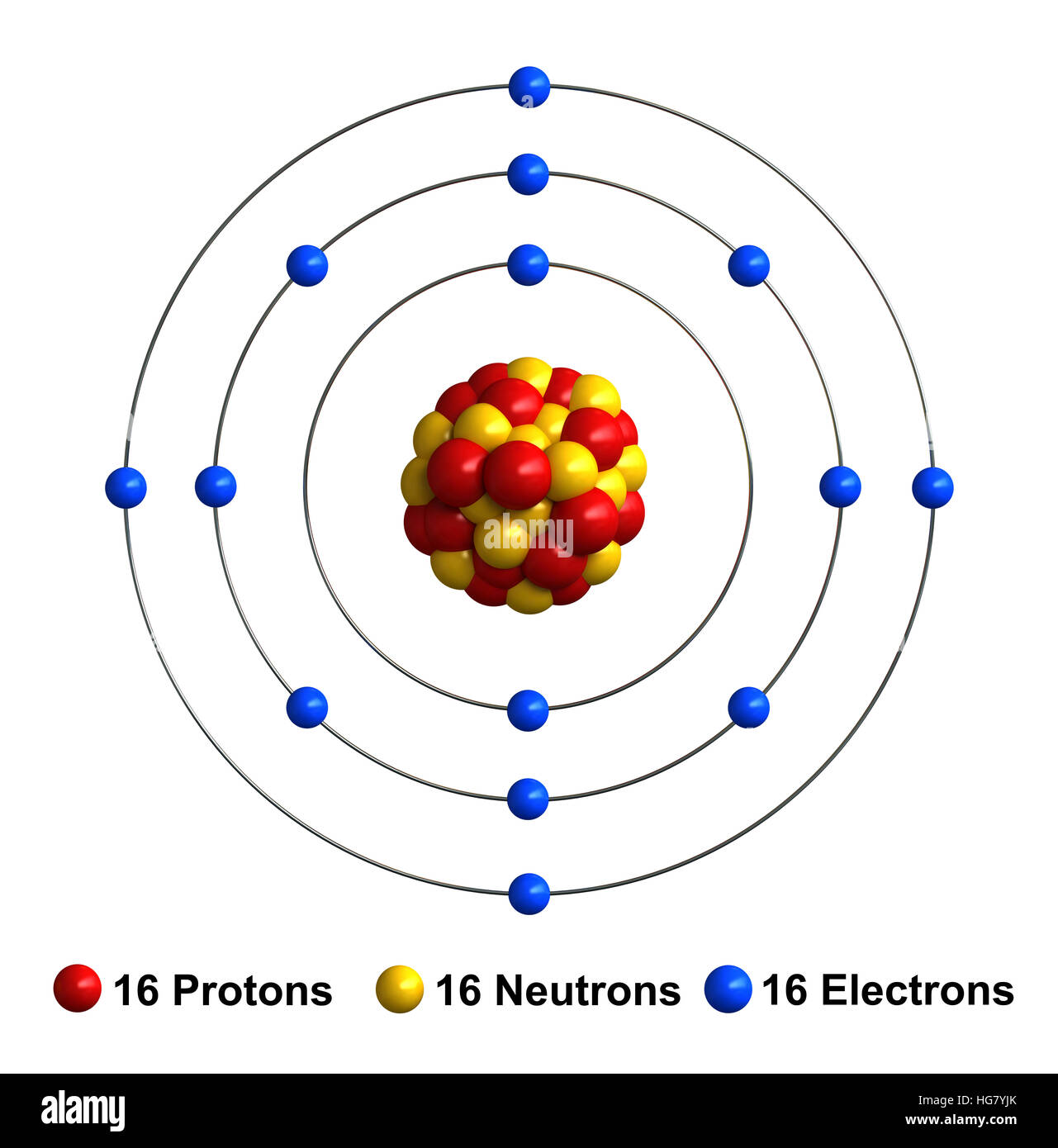
3d render of atom structure of sulfur isolated over white background
Diagram representation of the element sulfur Vector Image
Related Post:


:max_bytes(150000):strip_icc()/sulfuratom-58b602563df78cdcd83d5a9d.jpg)