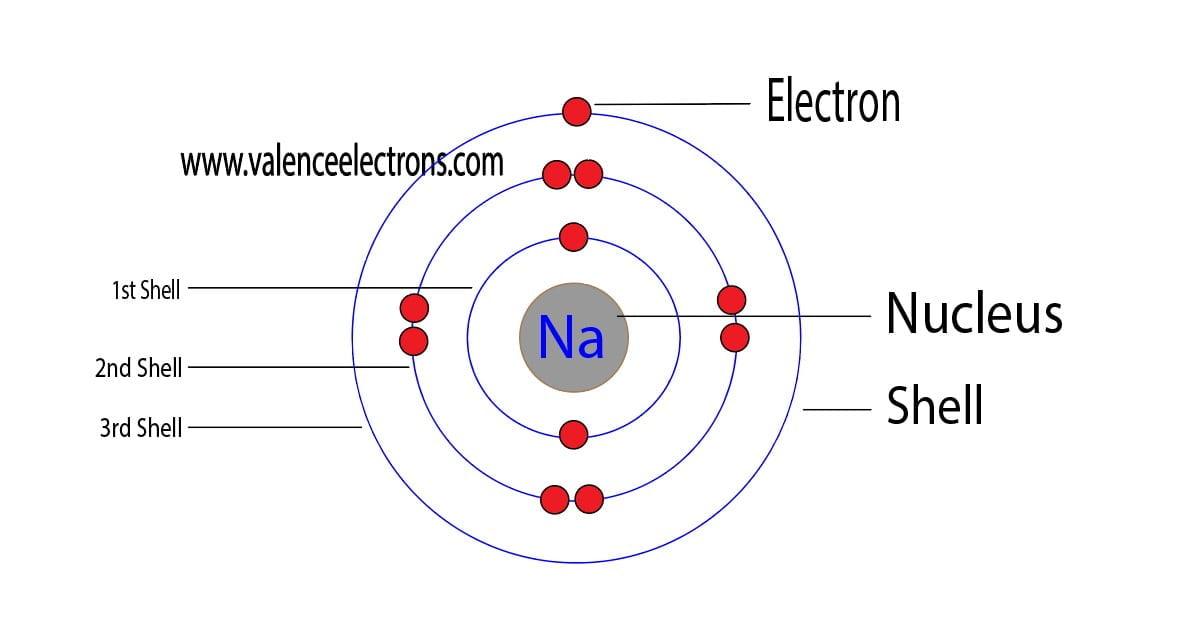
Sodium Electron Configuration Long Form
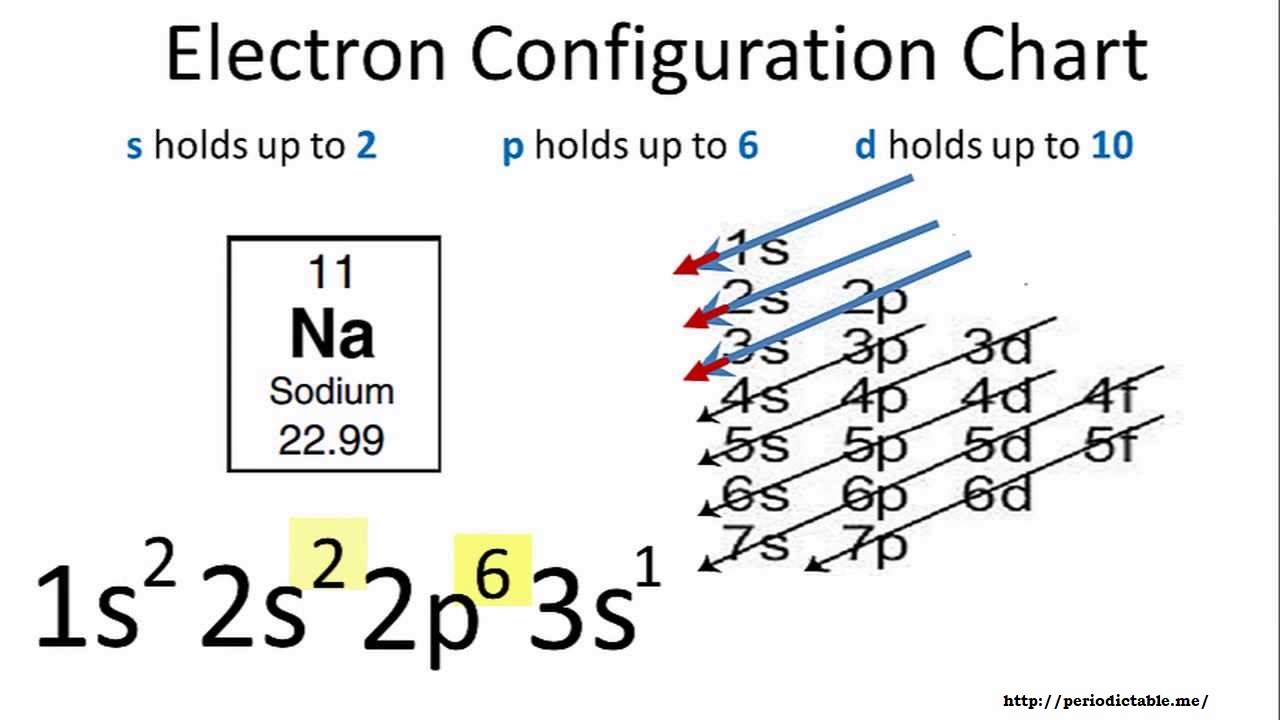
Sodium Electron Configuration Long Form - The number of electrons in sodium ion is 10. Since it is the outermost (valence) electrons which. Web the electron configurations and orbital diagrams of these four elements are: Sodium is one of the chemical elements that make up the periodic table, which is distinguished by an atomic number of. Electronic configuration of sodium in short form: A vertical column in the periodic table. Web this page shows the electron configurations of the neutral gaseous atoms in their ground states. Members of a group typically have similar properties and electron configurations in their outer shell. Web 1s^2 2s^2 2p^6 3s^1 since sodium is element number 11 on the periodic table, it implies a neutral stable na atom has 11 electrons surrounding its nucleus in the. The electronic configuration of sodium ion is given by. The alkali metal sodium (atomic number 11) has one more electron than the neon atom. Web electron configurations and the periodic table. Although drawing out each orbital may prove to be helpful in determining unpaired electrons, it is very time. Web electron configuration for sodium the history of sodium periodic table history identifiers list of unique identifiers for sodium in. Web electron configurations and the periodic table. 1s 2 2s 2 2p 6 3s 1. Although drawing out each orbital may prove to be helpful in determining unpaired electrons, it is very time. Web when chemists write down the really long electron configuration of an atom with a large atomic number (and thus lots of electrons), they’ll sometimes abbreviate the. The sodium atom (na) and si +3 , p. Web the sodium ion is formed by losing 1 electron. Web in writing the electron configuration for sodium the first two electrons will go in the 1s orbital. Web this page shows the electron configurations of the neutral gaseous atoms in their ground states. Sodium is one of the chemical elements. The sodium atom (na) and si +3 , p. Web the commonly used long form of the periodic table is designed to emphasize electron configurations. Since 1s can only hold two electrons the next 2 electrons for sodium go in the 2s. Since it is the outermost (valence) electrons which. Web electron configuration for sodium (na, and na+ ion) sodium. Web the commonly used long form of the periodic table is designed to emphasize electron configurations. The alkali metal sodium (atomic number 11) has one more electron than the neon atom. Web the electron configuration of iridium is much longer than aluminum. Web electron configuration for sodium (na, and na+ ion) sodium is the 11th element in the periodic table. Web the electron configurations and orbital diagrams of these four elements are: Web 1s^2 2s^2 2p^6 3s^1 since sodium is element number 11 on the periodic table, it implies a neutral stable na atom has 11 electrons surrounding its nucleus in the. For each atom the subshells are given first in concise form, then with all. Web the electron configuration. Since it is the outermost (valence) electrons which. Although drawing out each orbital may prove to be helpful in determining unpaired electrons, it is very time. 1s 2 2s 2 2p 6 3s 1. Web electron configurations and the periodic table. Sodium is a classified alkali metal element. Web the sodium ion is formed by losing 1 electron. Electronic configuration of sodium in short form: Web long form of sodium electron configuration: Web electron configuration for sodium (na, and na+ ion) sodium is the 11th element in the periodic table and its symbol is ‘na’. For each atom the subshells are given first in concise form, then with. Web the electron configuration of sodium is \(1s^2 2s^2 2p^6 3s^1\) (table \(\pageindex{1}\)). Web long form of sodium electron configuration: Web its electron configuration is \(1s^2 2s^2 2p^6 3s^1\). The alkali metal sodium (atomic number 11) has one more electron than the neon atom. The electronic configuration of sodium ion is given by. Web the electron configurations and orbital diagrams of these four elements are: For each atom the subshells are given first in concise form, then with all. The electronic configuration of sodium ion is given by. Web electron configuration for sodium the history of sodium periodic table history identifiers list of unique identifiers for sodium in various chemical registry databases sodium. Web electron configurations and the periodic table. Web electron configuration for sodium (na, and na+ ion) sodium is the 11th element in the periodic table and its symbol is ‘na’. Members of a group typically have similar properties and electron configurations in their outer shell. Web long form of sodium electron configuration: Electronic configuration of sodium in short form: Web the electron configuration of iridium is much longer than aluminum. Web the electron configurations and orbital diagrams of these four elements are: Web 1s^2 2s^2 2p^6 3s^1 since sodium is element number 11 on the periodic table, it implies a neutral stable na atom has 11 electrons surrounding its nucleus in the. Web electronic configuration for sodium (na) | spdf trick | chemistry | atomic number #11. The electronic configuration of sodium ion is given by. Web the sodium ion is formed by losing 1 electron. Web the electron configuration of sodium is 1s22s22p63s1. Web in writing the electron configuration for sodium the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for sodium go in the 2s. The number of electrons in sodium ion is 10. Since it is the outermost (valence) electrons which. 1s 2 , 2s 2,. 1s 2 2s 2 2p 6 3s 1. Elements are placed in order on the periodic table based on their atomic number, how many protons they have. Web the electron configuration of sodium is \(1s^2 2s^2 2p^6 3s^1\) (table \(\pageindex{1}\)).Sodium Electron Configuration Photograph by Photo
Electron arrangements
Sodium Electron Configuration (Na) with Orbital Diagram
Sodium electronic configuration How to Write Sodium electronic
Lewis dot structure How to write?
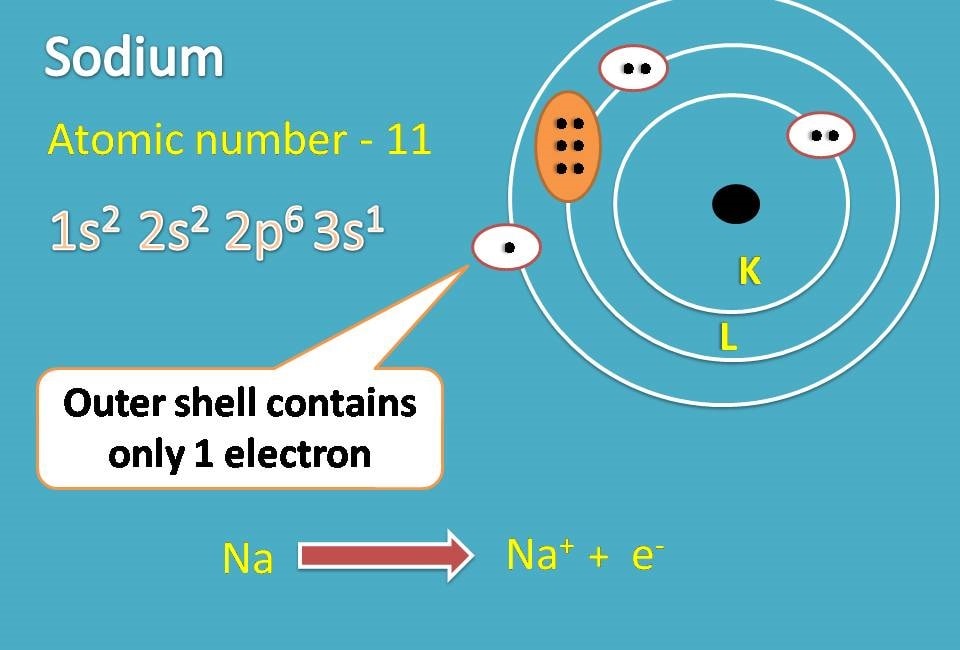
Distribution of Electrons in Different Orbits [with Examples] Teacho
Sodium Electron Configuration With Full Orbital Diagram
Diagram representation of the element sodium Vector Image
Electron Configuration for Sodium (Na, and Na+ ion)
Sodium Na (Element 11) of Periodic Table NewtonDesk
Related Post:
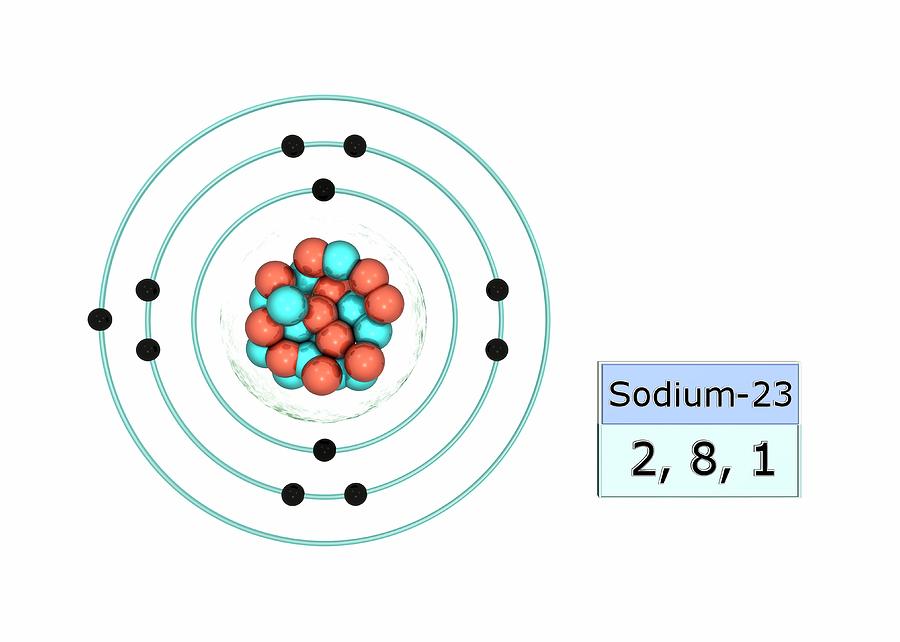
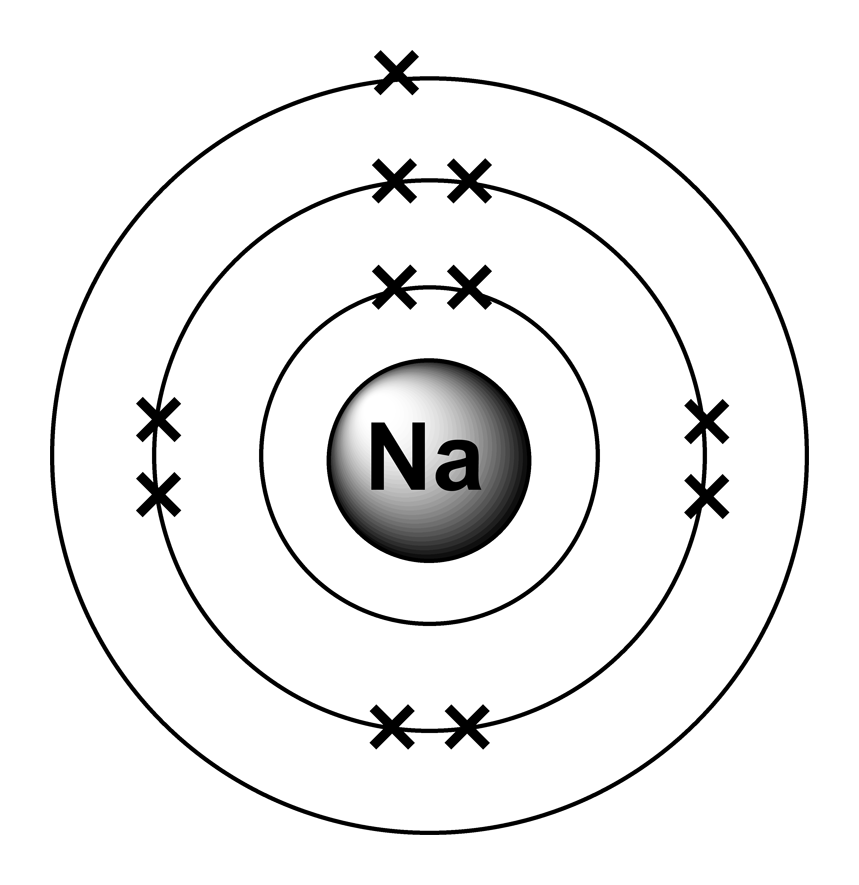

![Distribution of Electrons in Different Orbits [with Examples] Teacho](https://d1avenlh0i1xmr.cloudfront.net/a3108d07-47d4-4404-a645-de46563d93c2/17.-sodium-teachoo-01.png)